Water Decontamination Methods – Advantages and Disadvantages
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2597 words | ✅ Published: 28 Nov 2017 |
Outline
- Abstract
In Malaysia, for the past many years, drinking water treatment was only limited to water disinfection by using chlorine; however it had been concluded that its use in potable water treatment can be harmful to human health.(Subedi, et al., 2012) Following the development in drinking water treatment, conventional methods in three different technologies are available to have more purified water. They are biological, physicochemical and advanced oxidation process (AOPs) technologies. Each of the technologies has different roles in purifying water and they can be combined to treat drinking water as well. In this assignment, the lists of methods of each technology are stated and some of the main processes are discussed, including the advantages and disadvantages of each of them. For biological technology, slow sand filtration and perchlorate processes are discussed in 3a.For physicochemical technology, coagulation process is discussed in details in 3b. Lastly, the advanced oxidation process, AOPs, include both photochemical and non-photochemical oxidation. The fenton system and photocatalytic processes which are categorized in photochemical oxidation are discussed in 3c.
- Introduction
Safe drinking water that free of contaminants is essential to human health and development issue at national, regional and local levels. Its accessibility is human’s rights and a component of effective policy for health protection. (who et al) Therefore, there are many drinking water regulations and acts set by specialized agency such as WHO to have standard on safe drinking water to give awareness to public and thus limit the levels of contaminants. The national primary drinking water regulations and number of regulated contaminants shown in Figure 2.1 and Figure 2.2 in appendix 2.According to the regulations and standards, the characteristics of safe drinking water are contaminants free, natural minerals rich, alkaline pH, taste good and odorless. Firstly, microbiological and chemical contaminants in drinking water may cause acute or chronic health effects or undesirable aesthetic properties when present at excessive concentrations. Microbiological contaminants refer to pathogenic microorganisms such as bacteria, viruses and protozoan parasites. Chemical contaminants refer to toxicity, inorganic and organic chemicals. The other contaminants are pesticides, herbicides and radioactive materials (radionuclide). Besides that, pH of water should be maintained from 6.5 to 8.5. This is because alkaline water is able to neutralize stored acids and help in eliminating toxins. Because of these, various types of technologies of drinking water treatments are introduced to minimize the deleterious effects of contaminated drinking water on human health. Details of contaminants by National Secondary Drinking Water Standards and details of contaminants and potential health effects by National Primary Drinking Water Standards as shown in Figure 2.3 and Figure 2.4 respectively in appendix 2. In the following, three different technologies which are biological, physicochemical and advanced oxidation process (AOPs) are introduced to treat drinking water to a safety level so that human health is ensured. The goal of all of the developed water treatment technologies is to remove turbidity as well as chemical and pathogenic contaminants from drinking water source in the most affordable and expedient manner possible.
3.Content 3a.Biological Technology
Water used for drinking and household use, even water from a ground water supply, should be treated before it is used to ensure it is safe and aesthetically pleasing. One innovative method of water treatment is biological water treatment. Biological drinking water treatment is one technology that has the potential to further many of these objectives. This technology is based on the ability of microorganisms – specifically non-pathogenic bacteria – to efficiently catalyze the biochemical oxidation or reduction of drinking water contaminants and produce biologically stable water. (Snoeyink, 1984) .Biological drinking water treatment is often used in combination with other chemical and chemical processes including ozonation and filtration. There are some types of biological drinking water treatment such as slow sand filtration, rapid biological filtration, ozone-enhanced biological filtration and granular activated carbon biological adsorption.
Firstly, the slow sand filtration is a type of centralized or semi-centralized water purification system. A well-designed and properly maintained slow sand filter (SSF) effectively removes turbidity and pathogenic organisms through various biological, physical and chemical processes in a single treatment step. According to (Patrick J. Evans, 2010), slow sand filtration involves very low filtration rates (e.g. 0.04 to 0.10 gpm/ft2) through sand media without pre-oxidation or pre-disinfection (Awwa, 2005). During initial operation of slow sand filtration, a layer of biological matter will be produced on the surface of filter media by the separation of organic matter and other solids. This layer is called as schumutzdecke which acts as the predominant filtering mechanism. It supports the biological matter that works as the primary biofiltration process to remove BDOC, pathogenic microorganisms, and particulates (Page, 2006).To maintenance the slow sand filtration process, periodic scraping and removal of the top layer of sand are needed. Besides that, there is another more precise term to replace slow sand filtration which is called biological filtration (SBF) since the biologically active schmutzdecke is an integral part of this process. Besides that, the advantages of slow sand filters is very effective in improving the microbiological and physicochemical qualities of water and it is very easy to operate and maintain. The disadvantages of slow sand filters are vulnerability to clogging when the incoming water is of high turbidity. When dealing with such waters, pre-treatment, such as sedimentation or roughing pre-filtration is required.
Next, biological perchlorate or nitrate process. Perchlorate and nitrate have the ability of being anaerobically biodegraded to chloride and nitrogen gas. (Patrick J. Evans, 2010) stated the process involves addition of an electron donor such as acetic acid plus nutrients to water to promote biochemical reduction of biological perchlorate or nitrate process. Moreover, perchlorate and nitrate act as the terminal electron acceptors for respiration by these bacteria. As a result, BPNP vary from the predate biological drinking water treatment processes that are aerobic and employ aerobic bacteria that use oxygen as a terminal electron acceptor for respiration. BPNP can be employed in diverse arrangement including packed beds, fluidized beds, and membrane systems. BPNP is followed by an aeration process to promote aerobic biodegradation of assimilable organic (AOC) and biodegradable dissolved organic carbon (BDOC) in combination with a filtration process for turbidity removal.
3b.Physicochemical Technology
Physicochemical drinking water treatment is frequently used in the area of drinking water treatment. This technique is applied to remove the heavy metals, oils and suspended matters. This physiochemical drinking water treatment technique is used to treat drinking water in order to become process water. According to (Spellman, 2009)the steps that are under this technique are coagulation, flocculation, sedimentation, filtration, disinfection and arsenic removal from drinking water. The coagulation process that occurs in this drinking water treatment technique will be discussed in details in this assignment.
The definition of coagulation is the destabilization of colloidal particles.(L.Droste, 1997)The particles are coated with a chemically sticky layer that enables them to stick with each other, forming a large molecule and settle in a short period of time. The ability of an agent to agglomerate the tiny particles found in water is directly related to its charge. The other factor that will affect the ability is the size of synthetic polymers.
The most common materials that are used to coagulate the water are alum (aluminum sulfate) and iron salts. (L.Droste, 1997) stated that the multivalent characteristic of these coagulants effectively attracts them to charged colloidal particles and their high insolubility helps to ensure their removal from the water to a high degree.
When coagulant such as alum is being added into the water, a chemical reaction that produces positively charged 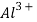 will occur. This reaction will indirectly reduce the electrical charges of the particles and hence form a sticky substance which also known as floc. In this reaction, turbidity, color and microorganisms can be removed easily. The formation of floc is the primary step of coagulation process. For perfect efficiency, intimate, rapid mixing of the water must be done and coagulant must be present. After mixing, the water should be stirred slowly so that the particles can stick to each other forming a large molecule.
will occur. This reaction will indirectly reduce the electrical charges of the particles and hence form a sticky substance which also known as floc. In this reaction, turbidity, color and microorganisms can be removed easily. The formation of floc is the primary step of coagulation process. For perfect efficiency, intimate, rapid mixing of the water must be done and coagulant must be present. After mixing, the water should be stirred slowly so that the particles can stick to each other forming a large molecule.
According to (Spellman, 2009) , the factors that will affect the coagulation process are pH, turbidity, temperature, alkalinity and he use of polymers. The degree to which these factors affect coagulation process relies on the type of coagulant use. The condition of raw water, optimum pH to coagulate the water and other factors must be taken into account before making decision on which chemical to be used.
According to(E.Manathan, 2009), the main advantage of using this technique is lower cost compared to biological treatment. It requires less worker force and able to settle the issues regarding toxic materials effectively. On the other hand, it has its disadvantage too, which is require careful operator control and use up huge amount of energy.
3c.Advanced Oxidation Process (AOPs)
Advanced oxidation process (AOP) is an oxidation process which affects water treatment by generating a sufficient quantity of hydroxyl radicals.(Goi, 2005) Hydroxyl radicals (• OH), are non-selective reactive species, helps to oxidize pollutants into mineral end-products, yielding CO2 and inorganic ions.This process can treat those organic pollutants with high chemical stability and low biodegradability which cannot be treated by conventional techniques. Basically, refractory compounds cannot be removed efficiently by conventional biological processes, but AOPs will do and help to improve the overall compound removal efficiency in water treatment.
|
Non-photochemical methods |
Photochemical methods |
|
– Ozonation at elevated pH (>8.5) – Ozone + hydrogen peroxide (O3/H2O2) – Ozone + catalyst (O3/CAT) – Fenton system (H2O2/Fe2+) |
– H2O2/UV – O3/H2O2/UV – Photo-Fenton/Fenton-like systems – Photocatalytic oxidation (UV/TiO2) |
Refers to Figure3.1 in appendix 2, in biological systems, AOPs are used as pre- and post-treatment. The pre-treatment improve wastewater biological treatability by common microorganism whereas post-treatment is targeted on the contaminants removal which not completely done during the biological treatment. (Cesaro, et al., 2013) Organic contaminants such as halogenated hydrocarbons (trichloroethane, trichloroethylene), pentachlorophenol (PCP), detergents, pesticides, etc can be destroying by this process easily. Besides, the inorganic contaminants (cyanide, nitrite, and sulfide) can be oxidized by this process.
Non-photochemical and photochemical methods are used to generating the OH radicals. These two methods are as shown as table below:
- Fenton system
In Fenton system, hydrogen peroxide (H2O2) acts as an oxidation agent. When hydrogen peroxide presents in excess, Fe (II) oxidizes to Fe (III) within few seconds or minutes and hydroxyl radicals will be generated. The reactions are as shown as below:
Fe2+ + H2O2 → Fe3+ + OH– +.OH
The catalyst used in this process is iron salts which generate ferric ion, Fe2+as the Fenton’s reagent. Besides, ozone (UV-light) and transition metal salts are used.Mostly, ironsalts are used as transition metal salts. (Munter, 2001)Under the UV-radiation, the felton’s reagent undergoes oxidation processes that utilize activation of H2O2, has high efficiency to treat the hazardous organic pollutants that present in water. (Albert, 2010)
Fenton reagent uses in wastewater treatment to convert the contaminants to harmless compound such as carbon dioxide and inorganic salts. This Fenton’s oxidation is the most efficient method in removing effluent toxidity and color compare to coagulation-flocculation process. It helps to decrease the rate of chemical oxygen demand (COD), aromatic compounds, and total polyphenols in the wastewater. However, Fenton process also can be combined with coagulation to reduce flocs settling time, amount of COD, and enhanced color removal.
Photo-Fenton-type oxidation is a process when Fe3+ ions added to H2O2/UV process, resulted Fe (OH)2+ complex in pH3 condition.
Fe3+ + H2O →Fe (OH)2+ + H+
Fe (OH)2+ ↔ Fe3+ + OH–
When Fe (OH)2+ will further decompose into .OH and Fe2+ ions with the presence of UV light.
Fe(OH)2+hv→ Fe2+ + .OH
This type of reaction very relies on UV irradiation to start up the .OH generation. Besides, this UV irradiation can mineralize organic pollutants completely. Efficiency of Fenton/Fenton-like reagents with UV irradiation can be increased by efficient use of light quanta and photo-reduction of ferric ion.(Munter, 2001)
- Photocatalytic oxidation
Photocatalytic oxidation is an alternative AOP method which introduced high energy (photons of ultraviolet light,UV) into the treatment system. Besides that, it is a potentially green chemistry drinking water treatment process. Throughout the whole process, no reagents are added and only relatively harmless catalyst TiO2 and sunlight (source of UV) is needed. Solid titanium dioxide, TiO2 is used as the photocatalyst leading to chain reaction for the production of HO. free radicals. (E.Manathan, 2009)
When the surface of TiO2 is irradiated with ultraviolet radiation, “holes” (h) are generated at sites where excited electrons (e–) are produced:
TiO2 +hv → TiO2 (h + e–)
The surface holes may take electrons from dissolved hydroxyl ion to produce reactive hydroxyl radicals on the TiO2 surface:
TiO2 (h) + OH– → OH.
Refers to theFigure 3.2 in appendix 2, solar disinfection (SODIS) and solar photocatalytic disinfection (SPCDIS) are appropriate technologies for water disinfection of Cryptosporidium oocysts at household level. Cryptosporidium species are protozoan parasites that infect humans and causes diarrheal disease by the food-borne or waterborne routes. Cryptosporidium is capable to survive in the environment for long periods and even treated water is not guarantee to be safe from these infective parasites. Therefore, SODIS and SPCDIS are introduced to deactivate these microorganisms to reduce the risk of infection.
SODIS and SPCDIS are both using the same method to reduce the oocyst viability. Both of them involve storing contaminated drinking water in transparent containers that are placed in direct sunlight before consumption.The disinfection effect of sunlight only occurs at temperatures exceeding 45 ℃. Therefore during cloudy conditions, this process may take a longer time to ensure the safety of the drinking water.
Photocatalytic disinfection, SPCDIS uses the non-toxic photocatalyst, TiO2 to enhance and accelerate the inactivation rate of the parasites. For SPCDIS, the photocatalyst particles would have to be removed after solar exposure and before consumption. Due to this additional step, the probability of compliance within communities in developing countries is low and lead to the inefficiency of the treatment.
In order to overcome this problem, this photocatalyst,TiO2 has been isolated onto some form of coated flexible insert, which would reside permanently within the SODIS reactor. Due to the effectiveness of the cheap, flexible insert coated with the non-toxic photocatalyst TiO2, the photocatalytic oxidation is recommended since it enhances the diminution of the oocyst viability by as much as 50%.
4.Conclusions
The three technologies in treating contaminated drinking water used different methods to purify water. Each of them has advantages and disadvantages. They have faced respective challenges in terms of added research and development. Some of the common challenges are techniques for effective removal of emerging contaminants, synthetic chemicals and pesticides, as well as problems in dealing with spills of chemicals in navigable rivers and lastly the development of sustainable treatment. The challenge involving technological development is the needs of economic, appropriate, relevant and sustainable developing technology. (Ray & Jain, 2011) In fact, three of the technologies can be combined to bring the greatest efficiency in water treatment process. For biological technology, the slow sand filtrationand perchlorate processes are discussed. The biological treatment is environmental friendly but the limitation of the processes are low effectiveness when the turbidity of water source is high. For physicochemical technique, the method discussed is coagulation, one of the challenges it meets is large amount of energy consumption; whereas its future prospect could be get use of solar energy which is environment friendly. For AOPs, fentonand photocatalytic process are discussed. Both of the processes still need a further research about the fundamental concepts and reaction mechanism. It is because there are still much remain to be done in terms of maximizing its efficiency, by enhancing the performance-related properties of oxide materials.
5.References
/abstract 1 /intro 2,3 *4+WHO *5+natural regulations *biological /physico 6,7,8 carson /aops9,10,11,12 /13,14,15,16,17,18 (6) /conclu19
1
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


