Use of Low-coordinate Metal Aryls in Small Molecule Activation and Catalysis
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2639 words | ✅ Published: 18 May 2020 |
Discuss the use of low-coordinate metal aryls in small molecule activation and catalysis.
Introduction
Over the last couple of years, the discussion and research into the use of low-coordinate metal aryls in small molecule activation and catalytic reactions, has remained to be quite challenging but has opened a key area to research more into. There is a vast interest into the reactivity of low-coordinate metal aryls, now that this area of synthetic inorganic chemistry has been furthered explored. [1][2] Deborah L. Kays and her team at the University of Nottingham have explored and researched a wide range of low-coordinate metal aryls in relation to m-Terphenyl ligands, which discusses two-coordinate m-Terphenyl complexes as well as three-coordinate m-Terphenyl complexes. Furthermore, her team also discovered more research into the stoichiometric reactivity using Cobalt and Iron which are commonly known as 3d transition metals and then further discussion into catalysis with low-coordinate systems. Deborah Kays research will be highlighted and referred to throughout this text. [2][3]
Transition metal such as iridium, palladium, rhodium and ruthenium have been well established when it comes to precious metal homogeneous catalyst and chemical synthesis. However, in recent years, researchers have seen challenges when trying to match or exceed the high activity and selectivity commonly seen with palladium, rhodium and ruthenium catalyst, in order to attempt to try and overcome the high activity and selectivity, a number of ligands and conditions would have to be explored to deal with the unstable nature of the these metals. [4] Precious metal catalyst has been truly impactful and have shown to be efficient for large number of applications, especially pallidum catalyst as it has been manufactured into electrical materials and pharmaceuticals which are industries that are constantly open to the idea new upcoming catalyst. [4] Nevertheless, there are a few issues that are raised when using these precious metal catalysis, one of them being the issues faced when trying to overcome high activity and selectivity due to the instability, another issue being the fact that these metals are not readily available and in turn can be really expensive to purchase (Figure 1), plus the fact that the toxicity of these metals are extremely harmful hence its undesirable factor. Due to the given problems of these precious metal catalyst, more viable, economically and environmentally friendly alternatives have been currently researched into. [5] A possible alternative to help solve this problem could be using Earth’s Abundant metals, these may include titanium, vanadium, chromium, manganese, iron, cobalt, nickel and copper. These elements are 3d first row transition metal and in addition happen to be the amongst the most abundant elements in the earths crust, which means these metals are very readily available which is far more feasible than the precious metal catalysts and far less expensive therefore this is the more feasible alternative option. 3d transition metals are seen as an important group of metals and this is due to their strong inter–atomic metallic bonding which leads these metals to commonly have high melting and boiling points, this can be seen as a great advantage as it allows high temperatures when experimenting if it is needed and will be able to do this without denaturing the metal catalysis. These 3d metals can also be catalysts due to the fact that there are temporary changes in the oxidation state of these transition metal ions. [6][7] Amongst these metals, Iron has been seen to be used vastly and has significant advantages compared to the precious metal catalyst, one advantage being that, iron (by mass) is the most abundant element on Earth but the fourth abundant in the Earth’s crust (Figure 2). The main reason why Iron (in large numbers) is readily available on Earth is because of its nuclear stability in stellar nuclear fusion reactions, so large quantities are able to be produced. [8]

(Figure 1) the monthly market prices of Palladium, Rhodium, Iridium and Ruthenium transition metals from over the last 19 years. [9]

(Figure 2) this graph above represents elements which are found in the Earth’s upper crust. In the shaded green sector, Iron is seen to be the fourth abundant element, also indicates iron is amongst the major industrial metal. [10]
Small molecule activation
Carbon monoxide (CO), carbon dioxide (CO2) and nitrogen gas (N2) are chemical small molecules but there is a real issue and challenge when it comes to the activation of these small molecules. These small molecules exhibit high bond strengths which makes it extremely hard to overcome the large bond barrier. However, the activation of these small molecules allows them to be extremely useful when it comes to reactions such as Fischer–Tropsch catalysis. In this text we will be focusing more on the activation and binding of the small molecule: carbon monoxide, in the presence of low-coordinate first-row 3d transition metal aryls. [3]
The first-row 3d transition metals allow ligands such as carbon monoxide (which is a neutral ligand) to be bounded to the metal centre to activate the small molecule and can therefore lead to further reactions. These transition metals can exhibit this binding power, and this is mainly because of their various oxidation states. [11]
More research as gone into the activation of carbon monoxide rather than nitrogen gas and carbon dioxide when looking into low-coordinate metal aryls in small molecule activation. From this we can see that m-terphenyl complexes have shown the most potential. These m-terphenyl ligand complexes can exist as two-coordinate or three-coordinate complexes. Therefore, these m-terphenyl ligands are on high demand as they can allow the isolation of high unsaturated 3d transition metal complexes which usually show very unfamiliar bonding and reactivity. In this example obtained by Deborah L. Kays and her team, they used Cobalt (II) complexes a and b (shown in Figure 3) and react the complexes with CO which they have described to ‘afford sterically encumbered ketones’. [3] These reactions were completed at room temperature and the two terphenyls Cobalt (II) complexes were coupled together with CO through insertion and intramolecular attack of flanking mesityl. [2] 12]

(Figure 3) the scheme above displays the reaction of Cobalt (II) m-terphenyl complexes when reacted with CO, to produced either a benzophenone or a keto-fluorenone at room temperature. [2]
Another example obtained by Deborah L. Kays and her team, in this example Iron (II) complexes were used where 2a and 2b complexes reduce CO with a complete cleavage of the C≡O bond (Figure 4). This reaction is the first example of a reductive cleavage of C≡O by a low-coordination Iron complex. The reaction is taken place at room temperature and 1 bar pressure. According to Deborah L. Kays the C4O2 ring and squaraines are forced out-of-plane because of the broken conjugation which is due to the steric bulk of aryl ring substituents. She describes that it causes less delocalisation into the aromatic ring, this results in an unusual high C=O Infrared stretching frequency (Figure 5) compared to other squaraines.
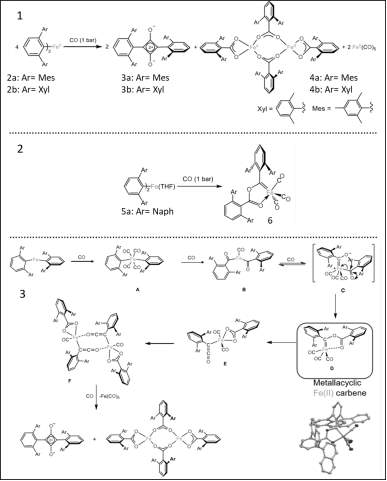
(Figure 4) the scheme above displays the reaction of Iron (II) m-terphenyl complexes when reacted with CO. 1) shows the squaraines formed when the substituted compounds Mes and Xyl react (2a and 2b), also the iron carboxylates formed (3a and 3b) and plus Fe(CO)5 compound formed. 2) shows the reaction of a three-coordinate complex (2,6-Naph2C6H3)2Fe(THF)) with CO to make iron-carbene, it is proposed that reaction stops at compound 6 because of an increase of flanking naphthyl groups which can prevent any further reaction. 3) shows the proposed mechanism for the reaction between 2a and 2b Iron complexes with CO. [2][3]

(Figure 5) the spectrum above displays compound 3a high but unusual C=O bond at 1673 cm-1. The IR spectrum also suggest that ketene intermediate might be formed during reaction. [2][3]
Catalysis
There is a great importance when it comes to the uses and wide applications of sustainable catalysts. In this case, as mentioned previously, chemists are moving towards using earth-abundant 3d first-row transition metals as they are cheaper and more readily available than palladium, rhodium and ruthenium. By using these earth-abundant transition metals, they will be able to exploit the high reactive complexes to accomplish a range of transformations.[3][4] One of the main reason why there is such a great interest with using low-coordinate systems in the aid of the catalyst is because they are able to act as catalyst precursors with coordinatively and electronically unsaturated complexes, therefore, the catalyst precursors have the innate electron deficiency at the metal centres and this can lead to unusual plus unique reactions and mechanisms. For example, rare isomerisation to Z isomer (Figure 6). [2]

(Figure 6) this scheme displays a rare isomerisation to Z in the presence of a low-coordinate metal aryl catalyst. [2]
Amine-borane has been in the spotlight as a great potential hydrogen storage material. Catalysed dehydrogenation reactions of amine-borane are thermodynamically favourable which makes the dehydrogenation process far more appealing for hydrogen storage systems. In addition to this, ammonia-borane has also gathered great interest into the potential of an onboard vehicular hydrogen delivery material as it is easily transported, can exist at normal temperatures and pressures. [2][13]

(Figure 7) This scheme above displays the cyclotrimerisation of isocyanates. a) shows the low-coordinate m-terphenyl metal complexes used as precatalysts in this reaction. b) this reaction is the general reaction for cyclotrimerisation of isocyanates. c) shows the homogenous mechanism. [3]
More recently, dehydrocoupling reaction have gathered vast interest as it provides cleaner and atom-efficient route to new bonds leading to the formation of dihydrogen. This has several applications in chemistry.[14] Manganese-based catalyst are rare however Deborah L. Kays and team were able explore more into this. They had an example where manganese complexes 7, 9 (Figure 7a) and xylyl-substituted analogue were able to catalyse dehydrocoupling of dimethylamine-borane (Figure 8). [3]
To sum up, they were able to find that two-coordinated catalyst 8 showed no significant changes when mercury was added and remained as a clear yellow solution, which lead them to believe that the reaction was completed via homogenous route. However when compared to the three-coordinated catalyst 9 it also showed a drop in activity but when 9 reacted with Me2NHBH3 there was a rapid change to a dark red suspension, this reaction with 9 led them to suggest that it is a ‘heterogenous in nature, involving the formation of catalytic nanoparticles’. [3]
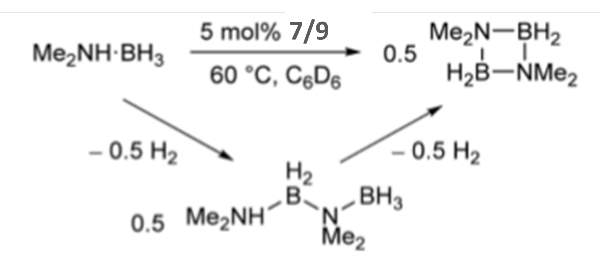
(Figure 8) this scheme displays the dehydrocoupling of Me2NHBH3. This reaction shows that there can be differs in reactivity when there are small changes in the steric properties of the flanking aryl groups of Manganese(II) m-terphenyl ligand complex. [3]
An active area of interest in chemistry is the investigation of catalyst that selectively catalyse cyclotrimerisation of isocyanates. [15] This is a very direct and atom-economical approach for the synthesis of isocyanurates. Deborah L. Kays and team have done recent work where they experimented with a series of Manganese(II) and Iron(II) m-terphenyl complexes were they found that these complexes were able to catalyse the cyclotrimerisation of isocyanates (Figure 7). This reaction supplies great application in coating material, medicines, drug-delivery and selective ion-bonding to name a few. They were able to find that 8 and 10 showed no notable difference in reactivity. However, found that 7 a two-coordinate complex, gives a much faster reaction then 9. They believed that the reaction for aryl isocyanates proceeded via homogenous pathway as they investigated the reaction stoichiometrically and found that there was no evidence for the formation of catalytic nanoparticles.
And lastly, we will be looking into the hydrophosphination of isocyanates with the aid of low-coordinate m-terphenyl catalyst complexes. When preparing phosphine there are major complications in terms of poor functional-group tolerance and side-product formation, which is a major disadvantage to the atom economy in the production if phosphine. Other methods are explored which are more favourable to atom economy. Which leads us to hydrophosphination as a synthetic route for phosphine products. According to this article by Helen R. Sharpe and co-workers explained that Iron(II) precatalysts when used to prepare phosphine can lead to a unique ‘catalytic double insertion of isocyanates into the P-H bond of diphenylphosphine to yield phosphinodicarboxamides’. However, there are still concerns with this method as it can lead to poisoning of the low-coordinated metal catalyst because of coordination capability of both phosphine substrates and products. [16] This example of the hydrophosphination of isocyanates is presented by Deborah L. Kays and team, they used Iron(II) m-terphenyl complexes 7 and 9 as ‘effective’ catalyst (Figure 7a). Monoinsertion and diinsertion of isocyanate into the P-H bond were achieved when the reaction took place. (Figure 9) They found that the three-coordinated 9 showed a much higher activity than 7, and they believed that this was probably due to the ‘presence of a liable THF ligand’. [3]

(Figure 9) This scheme above displays the low-coordinated Iron(II) catalysed hydrophosphination of isocyanates. a) shows the conditions when mixing of products in the hydrophosphination reaction. b) shows a change in conditions where selective formation is now achieved to produce monoinsertion (12) or diinsertion (13) products. c) shows a proposed catalytic cycle where the transition metal acts as a Lewis acid. [2][3]
Conclusion
To conclude, the discussion into the use of low-coordinate metal aryls in small molecule activation and catalysis has been greatly explored throughout this text with examples of mechanisms and schemes shown in Amine-borane hydrocoupling, Cyclotrimerisation of isocyanates and hydrophosphination of isocyanates. These have led to great applications of using low-coordinate metal aryls and especially using atom-economy earth-abundant transition metals. Although this field of chemistry is still in its early days, it still shows great promise and can definitely grow and develop further research in the future. This can be done by exploring more into 3d transition metals as there are vast numbers that have not be used in small molecule activation and catalyst.
References
- Clyburne, J. and McMullen, N. (2000). Unusual structures of main group organometallic compounds containing m-terphenyl ligands. Coordination Chemistry Reviews, 210(1), pp.73-99.
- Kays, D. (2018). Small molecule activation and catalysis using low-coordinate complexes.
- Taylor, L. and Kays, D. (2019). Low-coordinate first-row transition metal complexes in catalysis and small molecule activation. Dalton Transactions.
- Schafer, L., Mountford, P. and Piers, W. (2015). Earth abundant element compounds in homogeneous catalysis. Dalton Transactions, 44(27), pp.12027-12028.
- Enthaler, S., Junge, K. and Beller, M. (2008). Sustainable Metal Catalysis with Iron: From Rust to a Rising Star?. Angewandte Chemie International Edition, 47(18), pp.3317-3321.
- Docbrown.info. (2019). Introduction to 3d-block Transition Metal chemistry concepts definition data table characteristics variable ions oxidation states coloured compounds complexes catalysts high melting points high density GCE AS A2 IB A level inorganic chemistry revision notes. [online] Available at: http://www.docbrown.info/page07/transition1.htm [Accessed 27 Jul. 2019].
- Docbrown.info. (2019). Transition Metals compounds acting as catalysis catalytic theory & practice examples of homogeneous catalysts & heterogeneous catalysis GCE AS A2 IB A level inorganic chemistry revision notes. [online] Available at: http://www.docbrown.info/page07/appendixtrans06.htm [Accessed 29 Jul. 2019].
- Frey, P. and Reed, G. (2012). The Ubiquity of Iron. ACS Chemistry biology, (7 (9), pp.1477-1481.
- (Figure 1) Platinum.matthey.com. (2019). Price charts – PMM. [online] Available at: http://www.platinum.matthey.com/prices/price-charts [Accessed 2 Aug. 2019].
- (Figure 2) Izatt, R., Izatt, S., Bruening, R., Izatt, N. and Moyer, B. (2014). Challenges to achievement of metal sustainability in our high-tech society. Chem. Soc. Rev., 43(8), pp.2451-2475.
- Hagen, J. (2015). Industrial Catalysis: A Practical Approach. Focus on Catalysts, 2015(7), pp.17-46.
- Kays, D. (2017). Organic Transformations Catalysed by Low-Coordinate m-Terphenyl Complexes. [online] Available at: https://avestia.com/MHMT2017_Proceedings/files/paper/Keynote%20-%20Dr.%20Kays.pdf [Accessed 30 Jul. 2019].
- Bhunya, S., Malakar, T., Ganguly, G. and Paul, A. (2016). Combining Protons and Hydrides by Homogeneous Catalysis for Controlling the Release of Hydrogen from Ammonia–Borane: Present Status and Challenges. ACS Catalysis, 6(11), pp.7907-7934.
- Weetman, C., Ito, N., Unno, M., Hanusch, F. and Inoue, S. (2019). NHI- and NHC-Supported Al(III) Hydrides for Amine–Borane Dehydrocoupling Catalysis. Inorganics, 7(8), p.92.
- Wang, H., Li, H., Yu, X., Ren, Z. and Lang, J. (2011). Cyclodimerization and cyclotrimerization of isocyanates promoted by one praseodymium benzenethiolate complex [Pr(SPh)3(THF)3]. Tetrahedron, 67(8), pp.1530-1535.
- Sharpe, H., Geer, A., Lewis, W., Blake, A. and Kays, D. (2017). Iron(II)-Catalyzed Hydrophosphination of Isocyanates. Angewandte Chemie, 129(17), pp.4923-4926.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


