Synthesis Method of Napthoyl-thiourea Derivatives
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1646 words | ✅ Published: 26 Jan 2018 |
CHAPTER 3
METHODOLOGY
This chapter explains briefly on the synthesis method of napthoyl-thiourea derivatives, experimental characterization techniques (CHNS, FTIR, UV-Vis, UV-F, 1H and 13C NMR, Single Crystal X-Ray Crystallography Analysis, XRD, TGA, SEM, CV), electrodeposition of napthoyl-thiourea derivatives on ITO substrate, electrical conductivity measurement, IV curve characteristic, performance of organic diode and theoretical calculation via Gaussion 09 software package functions.
3.1 Reagents, Chemicals and Solvent
All chemicals used in this study were commercially available and used as received without any purification step taken. Chemicals namely 1-napthanoyl chloride (C10H7COCl), ammonium thiocyanate (NH4SCN), 2-aminopyridine (C5H6N2), 2-amino-5-chloro-pyridine (C5H5N2Cl) and 3-amino-4-methylpyridine (C6H8N2) were purchased from Sigma-Aldrich. In addition, 2-amino-5-picoline (C6H8N2) and 2-amino-3-picoline (C6H8N2) were supplied from Merck.
Solvents such as methanol (CH3OH), dichloromethane (CH2Cl2) and dimethylsulphoxide were supplied by Merck. Other solvents used in this study namely chloroform (CHCl3), ethyl acetate (CH3COOCH2CH3) and hexane (C6H14) were purchased from Sigma-Aldrich. Acetonitrile (C2H3N) and diethyl ether (C4H10O) were supplied by R & M chemicals. Whilst, acetone (CH3COCH3) was obtained from Fisher scientific.
3.2 Instrumentation and Characterization Techniques
The infrared (IR) spectra of the synthesized compounds were recorded on a Fourier Transform-Infrared Spectrophotometer, Perkin Elmer Spectrum 100 in the range of 4000-400 cm-1 using potassium bromide (KBr) pellets. Electronic absorption spectra of the samples were recorded in the range of 200-400 nm using Shimadzu UV-Visible Spectrophotometer 1601 series in 1cm3 cuvette while the electronic emission spectra were recorded in the same range by using Shimadzu UV-Fluorescence Spectrophotometer. For Single X-Ray Crystallography, the diffraction data were collected at 296.15K. The structure was solved and refined by using Olex2 solution program and refinement package using Gauss-Newton Minimization.
The 1H (400.11 MHz) and 13C (100.61 MHz) NMR spectra were recorded using Bruker Avance III 400 Spectrometer in CDCl3 as solvent and internal standard at room temperature in the range between δH 0–15ppm and δC 0–200ppm, respectively. Besides, CHNS-O Analyzer Flashea 1112 series was used to determine the experimental percentage of C, H, N and S elements of the synthesized compounds. Thermogravimetric analysis was performed using Perkin–Elmer TGA Analyzer from 0oC to 700oC at a heating rate of 10oC/min under nitrogen atmosphere. Analytical thin-layer chromatography (TLC) was carried out on precoated plate of TLC Silica Gel 60 F254 (Merck) and spots were visualized with ultraviolet light. The X-ray Diffraction (XRD) analysis was performed using Rigaku MiniFlexll from 3ï±-80ï±.
The technique cyclic voltammetry was performed using Electrochemical Impedence Spectroscopy (EIS) PGSTAT302 with three-electrode cell consisting of a polished Pt rod working electrode, Pt disc counter electrode and AgCl reference electrode. The solutions were ~10-3 M in electroactive material and 0.1 M supporting electrolyte, sulphuric acid. Electrochemical Impedance Spectroscopy (EIS) PGSTAT302 was used to coat the synthesized compounds on the ITO thin film by using electrochemistry method. EIS was used to study the oxidation and reduction state of the element of the synthesized compounds. The surface morphology of the final synthesised compounds in the form of powder and thin films were scanned by JSM 6360 Joel Scanning Electron Microscopy (SEM) with accelerated voltage 20 kV and magnification from 2000× until 10000× The electrical conductivity of the thin film was measured in the dark conditions by using Four-Point Probe and LI-200 Pyranometer Sensor with LI-1400 Data Logger while Keithley 4200 SCS Semiconductor Characterization System and Probe Station were used for efficiency determination and OLED parameters were evaluated from IV curve. The performance as Diode was performed by using D2 Power Supply GP-430D. Last but not least, the experimental result were proven by using ab initio quantum mechanical evaluation at the theoretical level of DFT B3LYP/6-31G (d,p). The physical parameters such as dipole moment, energy band gap (HOMO and LUMO) and Mulliken Charges Bond length were calculated using Natural Bond Orbital Theory at the same theoretical level.
3.3 General Research Design
In this study, the methodology is divided into four parts. Firstly, this research started with the synthesis napthoyl substructure of thiourea derivative. Secondly, all the synthesized compounds were characterized by using elemental analysis, typical spectroscopic and analytical techniques and electrochemical analysis. Next, the synthesized compounds were deposited on the ITO substrate as a thin film by using electrochemical deposition method. The conductivity and performance of the synthesized compounds as OLED were evaluated. Lastly, the experimental data were compared with the theoretical calculation by using Gaussion 09 software package. Figure 3.1 shows the schematic diagram of the experimental flow chart.
3.4 Synthetic Approach
The synthetic approach to obtain the compounds of interest is shown in Scheme 3.2. Thiourea derivatives (1–5) were prepared by using 1-naphthoyl chloride as starting material. The mixture of 1-napthhoyl chloride, an equimolar amount of ammonium thiocyanate and designated amines (3-methylpyridin-2-amine, 6-methylpyridin-3-amine, 5-methylpyridin-2-amine, pyridine-2-amine and 5-chloropyridin-2-amine) in acetone was put at reflux with constant stirring for around ca. 10 hours. After completion, the reaction mixture was cooled to room temperature before it was then filtered. The off-white precipitate was removed and the filtrate was added to some ice blocks. The precipitate was crystallised from hot acetone to afford crystals of the title compounds (1–5).

Scheme 3.2The general overview of the synthetic pathway applied in this study
3.5 Experimental Section
3.5.1 Synthesis of N-((3-methylpyridin-2-yl)carbamothioyl)-1-naphthamide (1)

A solution of 1-napthanoyl chloride (1.5ml, 1mol) with the equimolar amount of ammonium thiocyanate (0.76g, 1mol) in ca. 50ml acetone in 100ml two-necked round-bottom flask. The reaction mixture was put at reflux with continuous stirring for ca. 5 hours. Then, a solution of 6-methylpyridin-3-amine (1.07g, 1mol) in ca. 50ml acetone was added to the reaction mixture and was put at reflux with continuous stirring for ca. 7 hours. The progress of the reaction was monitored with TLC (Hexane: DCM; 3:2). Once the reaction completed the reaction mixture was cooled to room temperature and filtered into a beaker containing some ice cubes. The resulting light brown precipitate obtained, recrystallized from hot acetone to afford the title compound (1).
3.5.2 Synthesis of N-((5-metylpyridin-2-yl)carbamothioyl)-1-naphtamide (2)
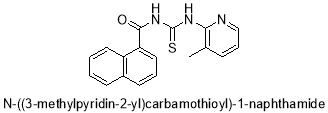
The title compound 2, (2.33g, 96%) obtained as light yellow crystalline solid was prepared from 1-napthanoyl chloride (1.5ml, 1mol), ammonium thiocyanate (0.76g, 1mol) and 3-methylpyridin-2-amine (1.07g, 1mol) in the manner as describe above.
3.5.3 Synthesis of N-((3-metylpyridin-2-yl)carbamothioyl)-1-naphtamide (3)

The title compound 3, (2.66g, 98%) obtained as light yellow crystalline solid was prepared from 1-napthanoyl chloride (1.5ml, 1mol), ammonium thiocyanate (0.76g, 1mol) and 5-methylpyridin-2-amine (1.07, 1mol) in the manner as describe above.
3.5.4 Synthesis of N-(pyridin-2-ylcarbamothioyl)-1-naphthamide(4)

In a manner similar to that described above 3, the title compound 4, (0.88g, 45%) obtained as light yellow crystalline solid was prepared from 1-napthanoyl chloride (1.5ml, 1mol), ammonium thiocyanate (0.76g, 1mol) and pyridin-2-amine (0.94g, 1mol).
3.5.5 Synthesis of N-((5-chloropyridin-2-yl)carbamothioyl)-1-naphthamide(5)

The title compound 5, (1.97g, 87%) obtained as light yellow crystalline solid was prepared from 1-napthanoyl chloride (3ml, 1mol), ammonium thiocyanate (0.76g, 9.95mmol) and 5-chloropyridin-2-amine (1.28, 1mol) in the manner as describe above.
3.6Electrodeposition of Naptoyl-Thiourea Derivatives on ITO substrate
The ITO substrates were used as working electrode was firstly cleaned with distilled water, detergent and acetone by using ultrasonic cleaner. All the synthesized compounds (1–5) were deposited on the ITO substrates by electrochemistry method by using Electrochemical Impendance Spectroscopy (EIS) PGSTAT302. By using the GPES method software, cyclic voltammetry (staircase) method was set to be in normal procedure. The depositions of the compounds were done in a mixture of compound dissolved in 45ml acetonitrile (10-3 M) and 5ml sulphuric acid (10-3 M) which act as supporting electrolyte .The cyclic of the process were set in Table 3.1 below. Figure 3.1 represent the arrangement layers of ITO/napthoyl-thiourea thin film. While, scheme 3.3 shows the overall methodology for organic light emitting diode (OLED) formation and characterization.
Table 3.1 Procedure set for electrodeposition process
|
No of scan |
5 |
|
Start potential (V) |
0 |
|
First Vertex Potential (V) |
0 |
|
Second Vertex Potential (V) |
3 |
|
Step Potential (V) |
0.01 |
|
Scan Rate (Vs-1) |
0.05 |

Figure 3.1The arrangement layers of ITO/napthoyl-thiourea thin film (1a–5a)
3.7 Electrical Conductivity Measurement
Four point probe was used to determine the conductivity of the thin film. The sheet resistivity of the films was measured with complete four probing system that consists of the Jandel Universal Probe combined with a Jandel RM3 Test Unit (Figure 3.2). In this study, the electrical conductivity of thin film was measured in dark condition to see its tendency to be applied as organic diode. Four probes were aligned and lowered onto the sample. The two outer probes supplied a voltage difference that drives a current through the film while the two inner probes pick up a voltage difference.

Figure 3.2Jandel Universal Probe and RM3 Test Unit
The sheet resistances (resistivity) for the thin films are shown in Equation 3.1 below. The unit of sheet resistance is ohms per square (ï-/sq):
Rs = 4.532 x V / I (Equation3.1)
Where: Rs = sheet resistance
4.532 = correction factor
V = voltage measured
I = current applied
The electrical conductivity can be determined which it is the reciprocal (inverse) of the electrical resistivity, ï³ as shown in Equation 3.2. The unit of electrical conductivity is ohm-1 m-1 (ï--1 m-1) = Siemens m-1 (Sm-1).
ï³ = 1 / Rs(Equation 3.2)
Where, ï³ = electrical conductivity
Rs = sheet resistivity
3.8 IV Curve Characteristic
The IV curves of the 1a–5a were measured by using Keithley 4200 SCS Semiconductor Characterization System and Probe Station (Figure 3.3). In this study, ITO substrate act as hole collecting layer (anode) while napthoyl-thiourea derivatives act as hole and electron carriers. The coated ITO substrates were mask with low work function metal which is aluminuim which act as cathode.

Figure 3.3Semiconductor Characterization System and Probe Station
The I-V curve shows the relationship between the currents and voltages gradient associated with the different current terminal (anode and cathode) of the diode. The obtained curve displays the forward current, reverse current, knee voltage and breakdown voltage of the diode.
3.9 Performance as Diode
The performances of 1a–5a as organic diode were tested by using D2 Power Supply GP-430D (Figure 3.4) under dark condition with difference voltagesin the range 15V to 30V.

Figure 3.4D2 Power Supply GP-430D
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


