Sulphuric Acid: Uses, Reactions and Environmental Issues
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2979 words | ✅ Published: 18 May 2020 |
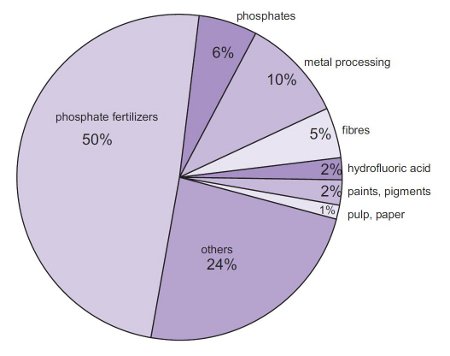
Sulphuric Acid (molecular formula: H2SO4) is one of the most important chemicals being produced and used worldwide having a wide variety of industrial and household usesas follows: *Refer to Appendix I and II*
Metal Processing:Used in the manufacturing of Copper and Zinc.
Medicines: Used in the manufacturing of Alkylating Antineoplastic agents
Pharmaceutical and Cosmetics: Many sulfonating agents are used in the manufacturing of dyes and pigments such as Titanium Dioxide and drugs used in the pharmaceutical and cosmetic industries.
Explosives: Used in the manufacturing of chemicals such as Nitric Acid which are used to make explosives.
Manufacturing of Fertilizers: It is used to make Phosphoric Acid which is required to make Phosphate fertilisers (the largest use of H2SO4) (Lazonby, 2016). Other types of fertilisers include: Superphosphate and Sulfates of Ammonia. 2NH3 (g) + H2SO4 (aq  (NH4)2SO4 (aq)
(NH4)2SO4 (aq)
Sulphuric Acid converts insoluble Calcium Phosphate, from phosphate rock, into mixtures that are soluble in water and therefore available for plants to absorb. The mixtures are crushed and used as Superphosphate fertilisers.
Dehydrating Agent: Sulphuric Acid is used to dry Chlorine gas produced by the electrolysis of a Sodium Chloride solution. It is also used as a drying agent in the manufacturing of explosives, dyes and detergents and is used as a catalyst in the dehydration of Ethanol to form Ethylene, the hydration of Ethylene to form Ethanol (used as fuel) and the condensation reaction between Alkanol and Alkanoic acid to produce Esters.
C2H5OH (l) 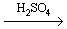 C2H4 (g) + H2O (l)
C2H4 (g) + H2O (l)
As an Electrolyte:Used as an electrolyte in Lead-acid batteries for motor vehicle engines.
Cleaning Impurities: Any grease or dirt from the surface of Iron or Steel is removed before galvanising or plating it with Zinc or Tin by treating it with acids such as Sulphuric Acid – this process is known as ‘pickling’. It is also used to wash impurities out of gasoline and other refinery products during petroleum refining. It is also used in the making of modern biodegradable synthetic detergents like Alkylbenzene Sulfonates.
Polymers Manufacturing: Sulphuric Acid is used in the production of many condensation polymers such as Rayon and Cellophane to extrude the liquid mass into fibrous polymer threads.
The Industrial Production of H2SO4: Production of Sulphuric Acid from raw materials is called the CONTACT PROCESS as during the formation of Sulphur Trioxide from Sulphur Dioxide, the reactants are kept in contact with a catalyst. There are four steps involved in the production of Sulphuric Acid at the industrial scale.
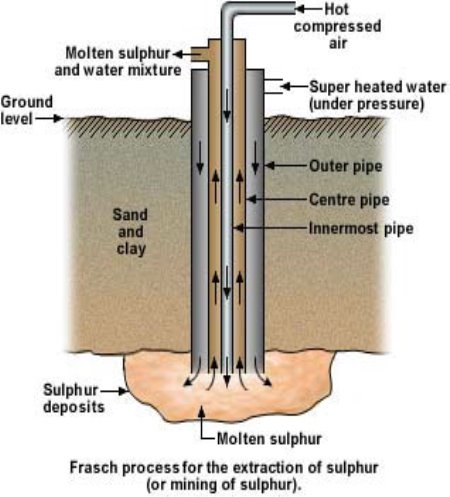
1) Extraction of Sulphur– is done to obtain the basic raw material (elemental Sulphur) needed for the contact process. Oxygen and Water are the other two reagents required for the process. *Refer to Appendix III*. The Frasch Process is used at various places to mine elemental Sulphur from underground mineral deposits (due to volcanic activity). The process involves three concentric pipes being drilled down into a Sulphur deposit. Superheated water (180°C under pressure) is pumped down the outside pipe melting the Sulphur which has a melting point of 115.2°C. Compressed air is pumped down the inside pipe, pushing the mixture of molten Sulphur and steam upwards through the middle pipe towards the surface (Scienceeasylearning, 2015). Sulphur is also found in ores such as Galena (PbS), as Hydrogen Sulfide in natural gas and petroleum and as Sulphates in the ocean. Sulphur is also released as Sulphur dioxide when metal sulfide ores are smelted. A general equation for this reaction, using M to represent a metal (such as Copper, Zinc or Iron), can be written as: MS + O2(g)  M(s) + SO2(g)
M(s) + SO2(g)
Sulphur is mostly produced as a by-product in the petroleum industry. The combustion of H2S produces SO2.
2H2S(g)+ 3O2(g)  2H2O(g) + 2SO2(g)
2H2O(g) + 2SO2(g)
This mixture is then cooled to condense Sulphur (boiling point 444.6°C).
Sulphur Dioxide Formation-Molten Sulphur (obtained through the Frasch process) is injected into a Sulphur burning furnace. Excess of dry air is pumped into the furnace at atmospheric pressure. The rate of reaction is increased by increasing the surface area of the molten Sulphur. The Sulphur burns in the presence of Oxygen, forming Sulphur Dioxide and heat as it is an exothermic reaction. S (l) + O2 (g)  SO2 (g) ΔH = -296kJ/mol
SO2 (g) ΔH = -296kJ/mol
This step involves the oxidation of Sulphur to Sulphur Dioxide. Itis an irreversible reaction (goes to completion) and proceeds rapidly in the furnace. The temperature of the SO2 gas and other unused air that comes out of the furnace is 1000oC, much of this heat must be removed before the second step. Sulphur Dioxide can also be obtained by heating Sulfide ores like Pyrite (FeS2) 4FeS2(s) + 11O2(g) → 2Fe2O3(s)+ 8SO2(g) or when Lead is extracted from Galena (PbS),
PbS(s) + O2(g) Pb(l) + SO2 (g). The Sulphur Dioxide formed as a by-product of the smelting process can also be used in the contact process and as a result of this, energy does not need to be expended to acquire it straight from Sulphur. Since Sulphur Dioxide is a pollutant, it should not be released into the atmosphere. Therefore, the location of the Contact plant should be near the site where smelting takes place. This will be efficient and cost-effective for both the industrial processes and protecting the environment from this pollutant.
Pb(l) + SO2 (g). The Sulphur Dioxide formed as a by-product of the smelting process can also be used in the contact process and as a result of this, energy does not need to be expended to acquire it straight from Sulphur. Since Sulphur Dioxide is a pollutant, it should not be released into the atmosphere. Therefore, the location of the Contact plant should be near the site where smelting takes place. This will be efficient and cost-effective for both the industrial processes and protecting the environment from this pollutant.
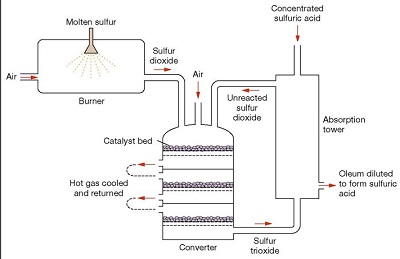
2) Conversion of Sulphur Dioxide to Sulphur Trioxide- The combustion furnace leads to the multi-stage catalytic SO2 conversion tower. Sulphur Dioxide is fed into this multi-stage catalytic converter, where it is converted into Sulphur Trioxide by a process known as catalytic oxidation.2SO2 (g) + O2 (g)  2SO3 (g) ΔH= -196kJ/mol
2SO3 (g) ΔH= -196kJ/mol
It is an exothermic and reversible reaction (reaches an equilibrium). The reaction conditions used are: Just above 1 atmosphere of pressure, 400-550°C, an excess of Oxygen and a catalyst Vanadium Pentoxide (V2O5) on porous silica pellets. The reaction proceeds in various stages of the converter until it reaches about a 99.7% conversion rate (*Refer to Appendix IV and V*). This step is performed in 2 stages using a catalyst.
(i) A fast but low yield stage at 550°C
(ii) A slower but high yield stage at 400°C
(i) Firstly, the temperature of the Sulphur Dioxide and the unused Oxygen gas mixture that comes out of the furnace is roughly 1000°C. It is cooled to 550°C by a heat exchanger and passed through the first bed of catalyst bed pellets, where a 70% conversion of SO2 to SO3 occurs rapidly (faster reaction rate). (Chemical book, 2017)
(ii) Since this reaction is exothermic, the Oxygen gas mixture is cooled again but to only 400°C this time. It is then passed through the second catalyst bed where almost 97% conversion to SO3 is achieved. Once again, the gas is cooled to 400°C and passed through the catalytic bed, where virtually all remaining SO2 is converted into SO3 or until a 99.7% conversion is achieved. This occurs at a slower rate but achieves a higher yield and the left-over residue gas at this stage is released back into the atmosphere. The excess heat produced from this exothermic reaction during step 1 and 2 can be reused to melt Sulphur in the Frasch process or to form steam for electricity production.
3) (a) Oleum Absorption:
When Sulphur Trioxide is dissolved in water, Sulphuric Acid forms. The overall reaction for Sulphuric Acid formation is:
SO3 (g) + H2O (l)  H2SO4 (g)ΔH=-227.72 kJ/mol
H2SO4 (g)ΔH=-227.72 kJ/mol
However, this reaction is extremely exothermic and results in a fog of corrosive and acidic Sulphuric Acid mist, which disperses in all directions, making it dangerous and difficult to handle. The process to separate the mist from the air is also too expensive to be conducted on an industrial scale. To overcome these issues, the Sulphur Trioxide is instead cooled in a heat exchanger and allowed to be absorbed by the 98% concentrated Sulphuric Acid in the Oleum production tank (also known as absorption tower), forming Oleum (H2S2O7) as shown by the equation. SO3 (g) + H2SO4 (l)  H2S2O7 (l)
H2S2O7 (l)
(b) Oleum Dilution:
The final product H2SO4 is formed conveniently by diluting Oleum (H2S2O7) with purified water in a dilution and storage tank. The Sulphuric Acid produced is 98% concentrated and of about 18M. The impurities released into the atmosphere at this stage only contain traces of Sulfur dioxide (0.3%). H2S2O7 (l) + H2O (l)  2H2SO4 (l)
2H2SO4 (l)
However, considerations must be taken into account for each individual step of this process on an industrial scale.
Temperature Considerations: Since the reaction to produce SO2 goes to completion, no temperature considerations are required. A high yield of SO3 could be achieved at low temperatures in step 3 but the rate of the reaction would be very slow. According to Le Chatelier’s principle, since the forward reaction is exothermic, reacting the gases at a lower temperature will shift the equilibrium to the right as the system attempts to counteract the lower temperature by producing more heat. However, the rate of reaction will also drop at a lower temperature. The rate of reaction will be greater at higher temperatures as the molecules gain energy, move faster and allow for more molecular collisions to take place however, the yield will be less. *Refer to Appendix VI* So, the temperature chosen is a compromise between reaction rate and yield. A temperature range of 400-550ºC is utilised, which provides a delicate balance between yield and rate of reaction. During the second passing through the catalytic converter the equilibrium will be shifted towards the right producing a greater yield by passing the gases at lower temperature in accordance with Le Chatelier’s Principle. This achieves almost a 97% conversion of SO2 to SO3.
Pressure consideration: Higher pressure shifts the equilibrium to the right creating more products (SO3) as there are fewer moles of gas in comparison to the reactants. By Le Chatelier’s Principle, increasing the pressure will shift the equilibrium in a direction to minimise the change by lowering the pressure hence creating more products (Yield). Also, increasing the pressure forces the molecules together and hence increases the concentration of the molecules. As a result of this the reaction rate also increases due to more molecular collisions taking place. Even though a high pressure will shift the equilibrium towards the right making more products, the equipment required is very expensive and dangerous; a compromise pressure of 1 or 2 atm is used which is enough to push the chemicals through for this reaction and even at these pressures there is a 99.7% conversion of SO2 to SO3 .
Concentration consideration: For the same reaction, increasing the concentration of reactants E.g. Oxygen causes the equilibrium to shift to the right creating more products/increasing yield in accordance with Le Chatelier’s Principle to minimise the change. The density of the reactant molecules increases by increasing the concentration. Therefore, the rate of the reaction increases due to greater chances of molecular collision. This is a cost-effective way of increasing the conversion of SO2 to SO3 as oxygen is readily available from the air.
Catalyst: The use of a catalyst has no effect on the equilibrium position but the conflict between rate and yield is resolved by employing a catalyst to increase the reaction rate enabling the reactions to proceed at lower temperatures to maximise yields. A catalyst of Vanadium Pentoxide (V2O5) on silica pellets greatly increases the rate of the reaction by lowering the activation energy, this is further aided by the increased surface area of catalyst by spreading it across multiple beds for the gases to react. This ensures that there is almost a complete oxidation of SO2 to SO3.
The Compromise: Finally, the compromise involves a moderate temperature (400-550ºC), use of a catalyst, using excess oxygen and a pressure not much above 1 atmosphere to have a fast-enough reaction rate and achieve the maximum possible yield. The compromise relating to pressure is more an issue of cost rather than rate or yield. Also, the temperature chosen and the catalyst used to achieve the desired rate and yield are efficient since the conversion is carried out in steps, one to achieve the fast rate and the other to increase the yield therefore there is no need for the purchasing of high pressure/temperature equipment. All the above conditions are chosen to give safety, an economically practical balance between the yield of products at equilibrium and the rate of the chemical reaction.
Likewise, the production of Sulphuric Acid has a significant impact on the environment, economy and also poses a range of social issues and therefore these influences are considered during the industrial production process. The chemical industry is growing and employs many people for various jobs such as engineers, chemists, truck drivers and accountants. Since Sulphuric Acid is used to make various types of household and industrial chemicals, leading to an expansive range of both uses and exports, many employment opportunities are created. An increase in jobs leads to an increase in household incomes. This further stimulates household spending, which holistically increases the quality of life of the population and increases the country’s economy. Also, the vast uses of Sulphuric Acid enhance living standards through the production of many day to day items, E.g. household cleaners improving sanitation and Sulphuric Acid being used in vehicle engines, improving vehicle performance and engine durability. Sulphuric Acid also aids in the production of fertilisers which reduces poverty and helps farmers produce crops efficiently thereby further increasing living standards. Furthermore, environmental impacts must also be considered, E.g. if the excess heat and superheated water used in Frasch process is discharged into the environment, it can cause thermal pollution due to its intense heat therefore it is recycled. As well as this, SO2 produced during smelting is sent to chemical plants to be used in the Contact process over being released into the atmosphere. Correspondingly, the extraction of large amounts of Sulphur during mining results in the formation of large underground caverns which can possibly collapse upon themselves resulting in land subsidence. While Sulphur itself is non-toxic and odourless, it easily oxidises to Sulphur Dioxide and reduces to Hydrogen Sulfide, both are dangerous air pollutants even at low concentrations; care must be taken to avoid these reactions. Sometimes soluble heavy metal compounds are brought to the surface by the Frasch process which can poison the ecosystem, food and water supplies. When Sulphur Dioxide combines with water and air, it forms Sulphuric Acid (all chemical equations shown in the contact process) which is the main component of acid rain. Acid rain can cause deforestation *Refer to Appendix VII*, affect ecosystems by disrupting food chains making them unbalanced, corrode limestone (CaCO3), marble buildings and paints. It can acidify waterways which is detrimental to aquatic life E.g. the exoskeleton of the crustaceans can be destroyed, leaving them defenceless. CaCO3 (s) + 2H+(aq)  Ca2+(aq) + H2O(l) +CO2(g).. Even small amounts of Sulphur Dioxide and acid rain can harm plants and trees by slowing down their growth, so farmers have fewer crops to harvest. “Sulphur Dioxide affects the respiratory system, particularly lung function by irritating the respiratory tract and can also irritate eyes.” (Qld.gov.au, 2019) Most of the Sulphur Dioxide released from metal sulfide smelters is used to make Sulphuric Acid in the contact process rather than being released into the atmosphere and causing environmental damage. SO2 emission into the atmosphere by industries is now strictly controlled by government regulations. Any spillage during production, storage, transport and use can cause major environmental issues especially if it comes in contact with water as the reaction is violently exothermic. The haulage centres should be located close to the plant so the transportation H2SO4 to the plant is cost effective and efficient. Scrubbers are also used to remove contaminants from gases before releasing them into the atmosphere. During transport, if there is a spill, certain methods need to be followed to neutralise the spill and prevent the area from being contaminated with Sulphuric Acid. Nowadays, Green chemistry is used with the aim to reduce the wastage, reduce the release of hazardous chemicals into the atmosphere and use of the renewable resources for energy production than the fossil fuels used for combustion reactions.
Ca2+(aq) + H2O(l) +CO2(g).. Even small amounts of Sulphur Dioxide and acid rain can harm plants and trees by slowing down their growth, so farmers have fewer crops to harvest. “Sulphur Dioxide affects the respiratory system, particularly lung function by irritating the respiratory tract and can also irritate eyes.” (Qld.gov.au, 2019) Most of the Sulphur Dioxide released from metal sulfide smelters is used to make Sulphuric Acid in the contact process rather than being released into the atmosphere and causing environmental damage. SO2 emission into the atmosphere by industries is now strictly controlled by government regulations. Any spillage during production, storage, transport and use can cause major environmental issues especially if it comes in contact with water as the reaction is violently exothermic. The haulage centres should be located close to the plant so the transportation H2SO4 to the plant is cost effective and efficient. Scrubbers are also used to remove contaminants from gases before releasing them into the atmosphere. During transport, if there is a spill, certain methods need to be followed to neutralise the spill and prevent the area from being contaminated with Sulphuric Acid. Nowadays, Green chemistry is used with the aim to reduce the wastage, reduce the release of hazardous chemicals into the atmosphere and use of the renewable resources for energy production than the fossil fuels used for combustion reactions.
APPENDIX:
Appendix I: (Lazonby, 2016)

Appendix II: (Chemical book, 2017)

Appendix III: (Scienceeasylearning, 2015)
Appendix IV: (Chemical book, 2017)

Appendix V: (QS Study, 2019)
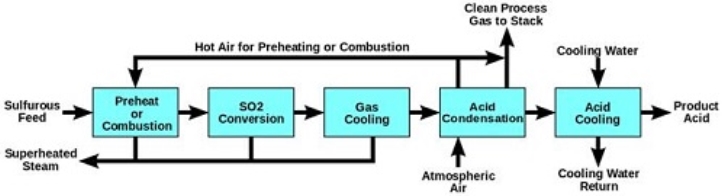
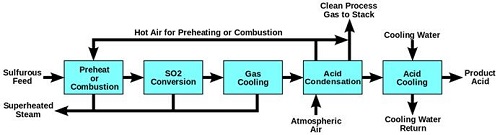
Appendix VI: (Dynamicscience.com.au, 2019)


BIBLIOGRAPHY:
Appendix VII: (Science and Earth, 2018)

BIBLIOGRAPHY:
- Chemguide.co.uk. (2019). The Contact Process for the manufacture of sulphuric acid. [online] Available at: https://www.chemguide.co.uk/physical/equilibria/contact.html [Accessed 1 Jul. 2019].
- Lazonby, J. (2016). Sulfuric acid. [online] Essentialchemicalindustry.org. Available at: https://www.essentialchemicalindustry.org/chemicals/sulfuric-acid.html [Accessed 28 Jun. 2019].
- Medium. (2014). Manufacturing of sulfuric acid by Contact process – WorldOfChemicals – Medium. [online] Available at: https://medium.com/@worldofchemical/manufacturing-of-sulfuric-acid-by-contact-process-be236265eaa2 [Accessed 30 Jun. 2019].
- Physicsservello.com.au. (2019). [online] Available at: https://physicsservello.com.au/files/contact process.pdf [Accessed 29 Jun. 2019].
- scienceeasylearning. (2015). FRASCH PROCESS FOR THE EXTRACTION OF SULPHUR. [online] Available at: https://scienceeasylearning.wordpress.com/2015/01/11/frasch-process-for-the-extraction [Accessed 1 Jul. 2019].
- Tsfx.com.au. (2019). [online] Available at: https://www.tsfx.com.au/wp-content/uploads/2014/07/mc2-2014-student-notes-y12-chemistry-sulfuric-final.pdf[Accessed 2 Jul. 2019].
- Dynamicscience.com.au. (2019). Chemistry-sulfuric acid-contact process. [online] Available at: https://www.dynamicscience.com.au/tester/solutions1/chemistry/sulfuricacid.html [Accessed 7 Jul. 2019].
- Encyclopedia.com. (2019). Sulfuric Acid | Encyclopedia.com. [online] Available at: https://www.encyclopedia.com/science-and-technology/chemistry/compounds-and-elements/sulfuric-acid[Accessed 4 Jul. 2019].
- Qld.gov.au. (2017). Sulfur dioxide | Environment, land and water | Queensland Government. [online] Available at: https://www.qld.gov.au/environment/pollution/monitoring/air/air-pollution/pollutants/sulfur-dioxide[Accessed 5 Jul. 2019].
- QS Study. (2019). Uses of Sulphuric Acid – QS Study. [online] Available at: https://www.qsstudy.com/chemistry/uses-of-sulphuric-acid[Accessed 1 Jul. 2019].
- Science, L. and Earth, P. (2018). Acid Rain: Causes, Effects and Solutions. [online] Live Science. Available at: https://www.livescience.com/63065-acid-rain.html [Accessed 7 Jul. 2019].
- Chemical book. (2017). Sulfuric ACid. [online] Available at: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB9675634.htm [Accessed 6 Jul. 2019].
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal