Structure of Hydrogen, Carbon, Oxygen and Nitrogen
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2142 words | ✅ Published: 23 Sep 2019 |
Molecules of Life P1, P2, M1
P1: Describe the structure of hydrogen, carbon, oxygen and nitrogen and relate these to the structure of biological molecules
M1: Explain the relevance of the electronic configuration of hydrogen, carbon, oxygen and nitrogen
Hydrogen
Hydrogen is used in petrol and diesel cars, the burning of fuel produce carbon dioxide and water. An element has one type of atom and it cannot be broken down into other substances. Most carbon compounds contain hydrogen and hydrogen forms compounds with all other elements. Compounds of hydrogen are normally called hydrides, even though the hydride describes compounds that have an H- ion.
(Carr and Carr, 2018) Hydrogen is the simplest form of atom; it has a nucleus like any other atom and the nucleus of the hydrogen is made of one proton and around the nucleus, there is one electron which goes round the nucleus. The proton particle has a positive electrical charge which is the opposite of the electron.
Because hydrogen has one electron, two hydrogen atoms will therefore share one electron between the two to form a covalent bond and this will make a hydrogen molecule.
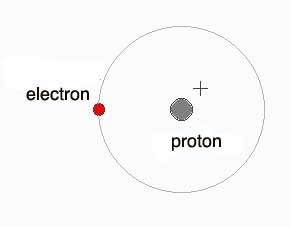
Picture: https://quatr.us/chemistry/hydrogen-atoms-chemistry.htm
Carbon
(Carr and Carr, 2018) Carbon is heavier than hydrogen atoms because they have 6 protons and 6 neutrons on the nucleus and 6 electrons going round the outside. For carbon there are two shells going around the nucleus because not all 6 electrons can go on around the nucleus at the same time, so 2 electrons can only fit in the inner shell and the rest on the other shell. With the outer shell, it can hold up to 8 electrons which make it easier for it to combine with other atoms to make even bigger molecules.
Carbon is known to be special compared to other atoms because it can bond with other carbon atoms to an unlimited extent; this is because it is small in size and can fit as part of larger molecules.
Hydrogen bonds can be replaced with another carbon atom covalently bonded to the first carbon atom. With this happening the long chains of carbon compounds can be made. Nitrogen, oxygen, and phosphorus can also bond with the carbon atoms, and these molecules form rings which then can link with other rings.
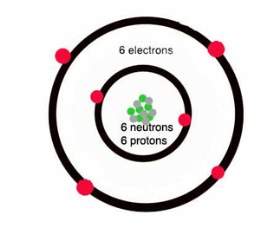
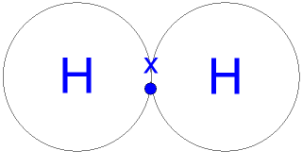
Picture: https://quatr.us/chemistry/carbon-atoms-chemistry.htm
Picture: http://www.gcsescience.com/a24-covalent-bond-hydrogen-gas-molecule.htm
Oxygen
Oxygen is an element that is formed by compounds by reaction with any other elements. All of the atoms are oxygen atoms but they do not exist separately so what would happen is that they would part up to form oxygen molecules.
(Gcsescience.com, 2018) Oxygen is heavier than hydrogen and carbon because of the number of protons, neutrons and electrons. An oxygen atom has 8 protons, 8 neutrons and 8 electrons around the nucleus. There are 2 electrons in the inner shell because that’s how much an inner shell can carry and 6 electrons on the outer shell. Two oxygen bonds will each share two electrons to form covalent bonds and make an oxygen molecule.
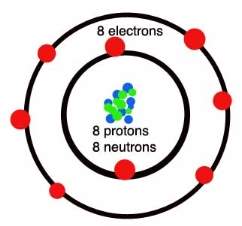
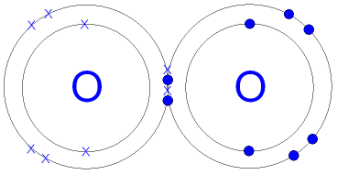
Picture 1: https://quatr.us/chemistry/oxygen-atoms-elements-chemistry.htm
Picture 2: http://www.gcsescience.com/a26-covalent-bond-oxygen-gas-molecule.htm
Nitrogen
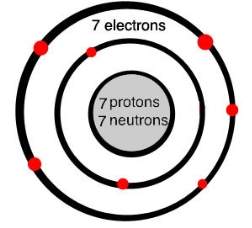
(Gcsescience.com, 2018) Nitrogen’s structure has seven protons, 7 neutrons and 7 electrons. 2 electrons on the inner shell and 5 electrons on the outer ring. What happens is that because there are 5 electrons on the outer shell, two nitrogen atoms will share three of the electrons to form three covalent bonds. This then makes a nitrogen molecule. By sharing six electrons on both sides of where the shells are touching, each of the nitrogen atoms can count eight electrons in the outer ring. Eight electrons on each atom show that the shared electrons are stable because the outer ring will be full.
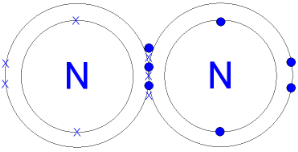
Picture: https://quatr.us/chemistry/nitrogen-atoms-elements-chemistry.htm
Picture: http://www.gcsescience.com/a25-covalent-bond-nitrogen-gas-molecule.htm
Biological Molecules
Carbohydrates, proteins, and lipids are biological molecules because they are built from smaller organic molecules. Each one of these has its own function because they are all an important component of the cell. Biological molecules are organic, this means that they contain carbon.
Carbohydrates
(Khan Academy, 2018) Carbohydrates are compounds that provide energy through a process called oxidation, they supple carbon for the synthesis of cell components, they serve as a form of chemical energy and they are part of the structures of some cells and tissues.
Carbohydrates are made up of carbon, hydrogen and oxygen, and the carbohydrates chains come in different length sizes and in three different categories: monosaccharides, disaccharides and polysaccharides.
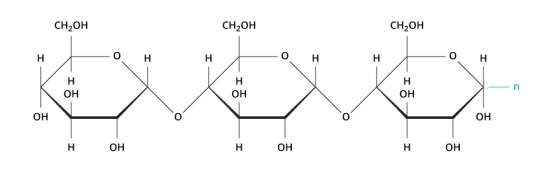
Picture: https://www.visionlearning.com/en/library/Biology/2/Carbohydrates/61
Proteins
(Reference, 2018) A protein is a biological molecule that is composed of polymers of amino acids joined together by peptide bonds. Proteins are different to carbohydrates and fats because it contains nitrogen. Proteins have many roles when involving the body; they do most of the work in the cells and are required for the structure and regulation of the body’s tissue and organs. They are about 20 different types of amino acids that are combined to make a protein.
(Mytutor.co.uk, 2018) Proteins function is to transport and store other molecules such as oxygen. There are four stages of making a protein. The first stage is called the primary structure is the order of amino acids in the peptide. Secondary structure is the folding of the polypeptide chain. The tertiary structure is the 3D folding, and finally quaternary structure is the protein being made.
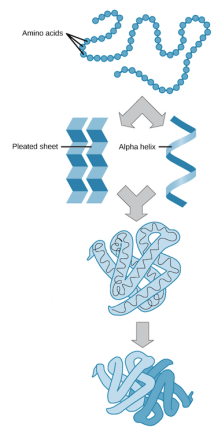
Lipids
(expii, 2018) Lipids are the other biological molecules that are made from long carbon chains that is bonded by hydrogen. Lipids are insoluble meaning that they cannot be dissolved in water however; they are soluble to non-polar molecules meaning that will dissolve. Lipids include fats, fat soluble vitamins, phospholipids and triglycerides. The functions of lipids is to serve structural components of biological membranes, they provide energy. Body fat is a good source of energy and it is stored as adipose tissues. They can be used to create hormones and steroids that the body uses to communicate from one cell to another cell and organ or organ.
(Torresbioclan.pbworks.com, 2018) The cell membrane is composed of proteins and lipids. The lipids help the membrane be flexible, the proteins help monitor and maintain and cells chemical climate, and it also transfers molecules across the membrane. Phospholipids have a head which is called a hydrophilic head and it has a tail called a hydrophobic tail. This means that the head is polar and the tail in non-polar. Phospholipids have a polar phosphate group which is hydrophilic which means it will face the aqueous solution because it is water loving. The fatty acid tails will move away from the aqueous solution because it id water—hating.
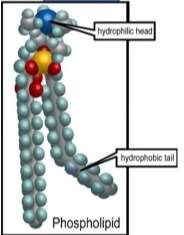
Picture: https://slideplayer.com/slide/8378455/
Picture: https://www.slideshare.net/sidrajaved2/lipids-62895056
P2: Describe the structure of water and carbon dioxide with reference to different types of bonding
Water
(Worldofmolecules.com, 2018) Water is a polar molecule and a chemical compound. A water molecule consists of two hydrogen atoms and an oxygen atom, its’ molecular formula is H2O. A water molecule is formed at an angle where the hydrogen atoms at the end and oxygen in the middle. Since oxygen has a higher electronegativity than hydrogen in general, the oxygen atoms have something called a partial negative charge. When this charge is so different, it is then called a dipole, and because of the charge difference, water molecules are attracted to each other. The relatively positive charged ones are attracted to the relatively negative ones. This attraction is called hydrogen bonding. The oxygen of one water molecule has two lone pairs of electrons; each of them can form hydrogen bonds with hydrogens on two other water molecules. This can carry on happening so that every water molecule is H bonded with four other molecules.
(Gcsescience.com, 2018) A covalent bond is formed when there is a sharing of an electron between two atoms. Water molecules have a covalent bond because there is a share of electrons that take place between the Hydrogen atom and the Oxygen atom and their electronic configuration. Hydrogen has one only electron in its outer shell and oxygen has eight electrons in its outer shell. What will happen is that the two hydrogen bonds will share their one electron with oxygen to form 2 covalent bonds and then this can make a water molecule. By sharing the two electrons where the hydrogen atom can count 2 electrons and oxygen can count eight. With hydrogen and oxygen sharing, it is now stable and the water molecule will not react with any other oxygen and hydrogen atoms.
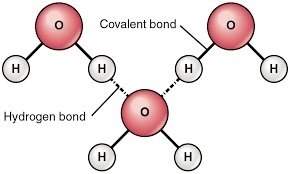
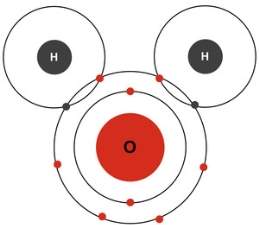
Picture: https://healingearth.ijep.net/water/structure-water
Carbon Dioxide
(Gcsescience.com, 2018) Carbon dioxide is a simple covalent molecule, its’ molecular formula is a carbon atom joined by two pairs of double bonds to the oxygen atoms O=C=O (CO2), carbon and oxygen are both non-metals. An oxygen atom has six electrons in its outer shell and a carbon atom has four electrons on its outer shell. The two oxygen atoms and 2 carbon atoms will share two electrons to form four covalent bonds, by doing this it will make a carbon dioxide molecule. With those four electrons being shared, each oxygen and carbon atom can then count eight electrons in its outer shell. Now the electrons on each atom are full of eight electrons, it is now stable and the carbon dioxide molecule will not react with any other oxygen or carbon atom.
The reason it is a double bond is because the carbon and oxygen has two bonds between the atoms. There are no ions present, meaning that there are no positive or negative charges in carbon dioxide gas. This is because the electrons are shared and not transferred from one atom to another.
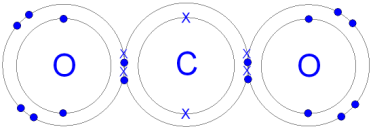
 #
#
Bibliography
CARBOHYDRATES
Khan Academy. (2018). Carbohydrates. [online] Available at: https://www.khanacademy.org/science/biology/macromolecules/carbohydrates-and-sugars/a/carbohydrates [Accessed 11 Dec. 2018].
CARR, K. AND CARR, K.
What is hydrogen? Atoms and Chemistry | Quatr.us Study Guides
Carr, K. and Carr, K. (2018). What is hydrogen? Atoms and Chemistry | Quatr.us Study Guides. [online] Quatr.us Study Guides. Available at: https://quatr.us/chemistry/hydrogen-atoms-chemistry.htm [Accessed 10 Dec. 2018].
CARR, K. AND CARR, K.
What is carbon? Atoms – Chemistry | Quatr.us Study Guides
Carr, K. and Carr, K. (2018). What is carbon? Atoms – Chemistry | Quatr.us Study Guides. [online] Quatr.us Study Guides. Available at: https://quatr.us/chemistry/carbon-atoms-chemistry.htm [Accessed 10 Dec. 2018].
CHEMICAL STRUCTURE OF LIPIDS – EXPII
expii. (2018). Chemical Structure of Lipids – Expii. [online] Available at: https://www.expii.com/t/chemical-structure-of-lipids-5531 [Accessed 12 Dec. 2018].
DESCRIBE THE STRUCTURE OF PROTEINS | MYTUTOR
Mytutor.co.uk. (2018). Describe the structure of proteins | MyTutor. [online] Available at: https://www.mytutor.co.uk/answers/3662/A-Level/Biology/Describe-the-structure-of-proteins/ [Accessed 11 Dec. 2018].
GCSE CHEMISTRY – COVALENT BONDING IN A CARBON DIOXIDE MOLECULE – WHAT IS THE STRUCTURE OF A CARBON DIOXIDE MOLECULE? – GCSE SCIENCE.
Gcsescience.com. (2018). GCSE CHEMISTRY – Covalent Bonding in a Carbon Dioxide Molecule – What is the Structure of a Carbon Dioxide Molecule? – GCSE SCIENCE.. [online] Available at: http://www.gcsescience.com/a27-covalent-bond-carbon-dioxide-gas-molecule.htm [Accessed 12 Dec. 2018].
GCSE CHEMISTRY – COVALENT BONDING IN A NITROGEN MOLECULE – WHAT IS THE STRUCTURE OF A NITROGEN MOLECULE? – GCSE SCIENCE.
Gcsescience.com. (2018). GCSE CHEMISTRY – Covalent Bonding in a Nitrogen Molecule – What is the Structure of a Nitrogen Molecule? – GCSE SCIENCE.. [online] Available at: http://www.gcsescience.com/a25-covalent-bond-nitrogen-gas-molecule.htm [Accessed 10 Dec. 2018].
GCSE CHEMISTRY – COVALENT BONDING IN A WATER MOLECULE – WHAT IS THE STRUCTURE OF A WATER MOLECULE? – GCSE SCIENCE.
Gcsescience.com. (2018). GCSE CHEMISTRY – Covalent Bonding in a Water Molecule – What is the Structure of a Water Molecule? – GCSE SCIENCE.. [online] Available at: http://www.gcsescience.com/a30-covalent-bond-water-molecule.htm [Accessed 12 Dec. 2018].
GCSE CHEMISTRY – COVALENT BONDING IN AN OXYGEN MOLECULE – WHAT IS THE STRUCTURE OF AN OXYGEN MOLECULE? – GCSE SCIENCE.
Gcsescience.com. (2018). GCSE CHEMISTRY – Covalent Bonding in an Oxygen Molecule – What is the Structure of an Oxygen Molecule? – GCSE SCIENCE.. [online] Available at: http://www.gcsescience.com/a26-covalent-bond-oxygen-gas-molecule.htm [Accessed 10 Dec. 2018].
REFERENCE, G.
What are proteins and what do they do?
Reference, G. (2018). What are proteins and what do they do?. [online] Genetics Home Reference. Available at: https://ghr.nlm.nih.gov/primer/howgeneswork/protein [Accessed 11 Dec. 2018].
THE WATER MOLECULE
Worldofmolecules.com. (2018). The Water Molecule. [online] Available at: https://www.worldofmolecules.com/solvents/water.htm [Accessed 12 Dec. 2018].
TORRESBIOCLAN / CELL MEMBRANE – TEAM SMART
Torresbioclan.pbworks.com. (2018). torresbioclan / cell membrane – team sMART. [online] Available at: http://torresbioclan.pbworks.com/w/page/22377072/cell%20membrane%20-%20team%20sMART [Accessed 12 Dec. 2018].
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


