Shellac for Film Formation and its Modification
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2484 words | ✅ Published: 28 Nov 2017 |
Shellac for film formation and its modification for enhancement of properties
Abstract
With the growing environmental concerns associated with synthetic polymers, the need for biopolymers has strongly emerged which can have applications ranging from packaging to electrical applications. Shellac, which is a naturally occurring product resin obtained from insects (female lac bug), is one such component which can be used for production of biopolymeric film. However, due to limitations on mechanical and barrier properties, modification of Shellac is necessary. Following is a reviewed study of modifications of Shellac to improve its film properties, heat resistance, water resistance, gloss and hardness.
Keywords:
Biopolymers, Shellac, modification, coatings.
1. Shellac
With the decrease in petroleum reserves, the need for biocompatible polymers has emerged. Polylactic acid, Zein protein films and Shellac, as in this case, have become vital for environmental concerns. Shellac is a naturally occurring polymer, obtained from resinous secretions of lac insects, Laccifer Lacca (Soradech et al) 1. Shellac as a coating material is mainly used in the fields of food and pharmaceutical industries (Jinwei Wang, Lei Chen, Yedong He) 2. However, one of shellac’s problems is a lack of solubility in commonly used coating solvents such as ketones and glycol ethers (Jennifer T. Otto, David L. Trumbo) 3. Problems associated with shellac are batch-to-batch variation, need of organic solvent, less stability and less solubility in alkaline pH of intestine, comparing to synthetic and semi-synthetic enteric polymers.
Shellac is primarily used as a naturalprimer,sandingsealant,tannin-blocker,odour-blocker,stain, andhigh-glossvarnish.
- Applications of Shellac
Due to excellent film forming and protective properties, it is widely used in food industry, paint industry and to a considerable extent in pharmaceutical industry. Use of biopolymers for packaging applications is severely limited due to poor barrier and mechanical properties (Melissa Gurgel Adeodato Vieira et al) 4. Significant work was carried out by (Hult et al) 5 by using Microfibrillar cellulose (MFC), incorporation of which reduces the Oxygen Transmission Rate (OTR) value (Syverud et al)6 combined with Shellac to produce fibre based packaging. Significant oxygen barrier for packaging was not obtained but multilayer coating with MFC and Shellac reduced OTR greatly. (D. Phan Te et al) 7 presents another way of eliminating the limitations of biopolymers for packaging. Formation of hydrocolloid-shellac bilayer films produced an enhanced resistance to water and moisture permeability with good mechanical properties. Better mechanical properties and better adhesion to the hydrocolloid layers was obtained with the use of plasticizer. Shellac has also been used widely in Pharmaceutical and drug retention applications. (Berg et al) 8 describes shellac as one of the very few materials to be used as a pharmaceutical coatings. It finds special applications in health applications and nutraceuticals (Krause et al) 9.
2. Modifications of Shellac for enhancement of properties
Modification has been carried out to overcome many popular limitations of Shellac films like low heat resistance, poor water resistance, poor solvent resistance, chemical resistance, brittleness, etc. (Sharma et al.) 10
2.1 Crosslinking of acetoacetylated Shellac with multifunctional amine or acrylate (Jennifer T. Otto, David L. Trumbo) 3
Crosslinking of films was carried out by reaction between acetoacetylated shellac with a multifunctional amine or with a multifunctional acrylate under Michael addition reaction parameters (Jennifer T. Otto, David L. Trumbo et al.)3. According to (Jennifer T. Otto, David L. Trumbo et al.)3 Shellac/t-butylacetoacetate films were synthesised for this purpose. The glass transition temperature of the modified shellac was observed to be 4.5°C. The Tg of unmodified shellac is 54.6°C.High level of acetoacetylation is indicated to have occurred as acetoacetylation is known to reduce Tgs by reduction in hydrogen bonding capabilities (Clemens, RJ, Rector, FD et al)11. Major purpose was to alter the solubility of the shellac. Modified Shellac was attempted to dissolve in various solvents. It was noted that the modified shellac was soluble in MEK, methyl amyl ketone (MAK), acetone, CHCl3, dipropylene glycol monomethyl ether (DPM), THF, and DMAC. The unmodified shellac was soluble in ethanol and DMAC and sparingly soluble or insoluble in MEK, CHCl3, MAK, and DPM. Thus, desired modification was obtained, and (Jennifer T. Otto, David L. Trumbo) 2 formed thermoset films crosslinked with two different species, an amine and a multifunctional acrylate. It is possible to modify shellac by reaction with t-butyl acetoacetate. This modification changed significantly different solubility characteristics and the ability to be crosslinked with different species at different temperatures, including ambient temperature. The crosslinked films have well to excellent properties, with the exception of low angle gloss for the DYTEK A cured films. This could reflect some incompatibility on a microscale between the modified shellac and the diamine. Gel content measurements suggest that a high degree of crosslinking has been obtained.
2.2 Modification by use of Maleated Shellac (Hasmukh S. Patel and Sumeet J. Patel) 12
Following modification was employed for coating preparation of maleated shellac-acrylic resin emulsion paints, acrylic resin its application as surface coating materials and characteristic. According to (Hasmukh S. Patel and Sumeet J. Patel) 12, maleated Shellac was synthesised by treating Shellac with Maleic Anhydride. Shellac solution in tetrahydrofuran (THF), different proportions (10–30% wt of shellac) of maleic anhydride was added and well mixed. The resultant reaction mixture was refluxed for 5 h before cooling and pouring into cold water. Adhesion and smooth finish film were obtained and no observable damage and detachment of the film was observed. Blending of Maleated Shellac with acrylic resin might give rise to the polymerization and/or crosslinking between the two components through the functionality due to unsaturation which resulted in better water resistance and decent alkali resistance even though shellac and becomes easily soluble when it comes in to contact with water and alkali, respectively. The coated panels were immersed by (Hasmukh S. Patel and Sumeet J. Patel) 12 in water for 5 months and no detachment or discolouration was observed. On the coating after taking out the panels from water. It was noted that water that was socked by the panels, evaporated kept at room temperature. Deterioration water socked panels of the coating was not observed which indicates that the composition resisted uphold of the water/ moisture within the system which is an important feature of the paint formulation.
2.3 Blending of Shellac with Epoxy resin (D. N. Goswami and S. Kumar) 13
The reaction represented below was employed as a modification for Shellac.

Figure 1: Shellac and Epoxy group reaction (D. N. Goswami and S. Kumar) 13
The above reaction represented in Figure-1 is a reaction mechanism between Shellac and Epoxy group. This reaction was monitored by reducing acid value or reducing epoxide value. The properties obtained were found to be optimum for cured samples containing 70 parts of shellac and 30 parts of the epoxy resin
(Tripathi et al) 14. Following figure (Fig. 2) shows the variation in dissipation factor (tan δ) for various shellac-epoxy resin blends with time.

Figure-2:Variation of dissipation factor (tan δ) of shellac-epoxy resin varnishes with time. (1) Dewaxed lemon (DL) shellac: epoxy resin = 60:40, mol. wt. of epoxy resin 500; (2) dewaxed lemon (DL) shellac: epoxy resin = 70: 30, mol. Wt. of epoxy resin 1 OOO; (3) dewaxed orange (DO) shellac: epoxy resin = 50: 50, mol. wt. of epoxy resin 1 OOO.
(D. N. Goswami and S. Kumar) 13
The dissipation factor undergoes an initial rise up to around the sixth day after blending and remains almost constant thereafter.
The variation of conductivity of different shellac-epoxy resin blends with time is shown in Fig. 3.
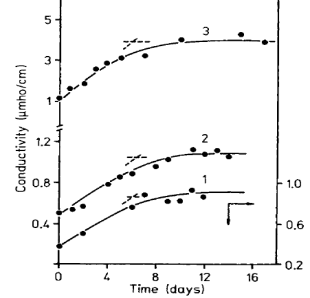
Figure-3: Conductivity variation with time (D. N. Goswami and S. Kumar) 13
Conductivity, like dissipation factor, also showed an initial increase with time. Around the sixth day, the variation was found to be biphasic in nature with an inflection. For the blends containing epoxy resins with molecular weights of 500 and 1000 the nature of variation of both dissipation factor and conductivity with time was found to be similar.
The variation of specific viscosity of the 70: 30 and 50: 50 blends with time is shown in Fig. 4.

Figure 4: Variation of specific viscosity of dewaxed lemon (DL) shellac-epoxy resin varnishes (D. N. Goswami and S. Kumar) 13
It was observed that initially specific viscosity increases with time and becomes almost constant after six days. A small plateau was observed for the next few days and thereafter specific viscosity increases. It was observed that the values of dissipation factor, conductivity, dielectric strength and specific viscosity of the epoxy resin-free shellac solutions were more or less constant.
If the reaction is carried for a longer period, besides the reaction mechanism as shown previously, cross-linking occurs. In this process, the hydroxyl groups of shellac produced by the above mentioned reaction probably react with the epoxide group and/or with the carboxyl group forming a three-dimensional network as shown in Fig.5

Figure 5: Reaction between Hydroxyl group and/or Epoxy or Carboxylic group. (D. N. Goswami and S. Kumar) 13
2.4 Combination of Shellac and Polyamidoamine (R. K. Dey, G. S. Tiwary, Tanushree Patnaik, Usha Jha) 15
The modification mentioned below was essentially done for drug delivery applications. Natural biodegradable and biocompatible polymer is encouraged as a starting material for synthetic purpose so as to reduce the production of the toxic biodegradable products in body’s physiological environment. By varying the ratio, a wide range of polymers were prepared. Shellac: PAA as 1: 1, 1: 2, and 1: 3 ratios was taken though the characterization was done by taking Shellac and PAA in the ratio 1: 1. According to (R. K. Dey, G. S. Tiwary, Tanushree Patnaik, Usha Jha) 15, the samples were prepared using the appropriate amount of PAA dissolved in 20 mL of methanol, added to a solution of Shellac in methanol. A solution of 2, 2-dimethoxy-2-phenyl acetophenone (about 2 wt % with respect to the PAA) was added in methanol (around 5 mL) to this mixture with mild stirring. The reaction mixture was poured into a glass petridish and was kept at room temperature. The polymerization was initiated by irradiation with an incandescent broad-spectrum lamp (Philips Comptalux, 150 W), positioned 25 cm above the petridish. Irradiation was continued for 7 h until gelation occurred. The schematic sketch of the reaction leading to the formation of polymeric material is shown in Figure 6. The polymeric material was extensively washed with methanol to remove any residual monomer, then freeze-dried and stored until further use. The resultant product was cut in films, dry in air for three days, and place in a vacuum oven at 25°C until constant weight. It was observed that PAA was soluble in water where as the corresponding polymer of Shellac-PAA was insoluble in water.

Figure-6: Synthesis of Shellac-PAA by photopolymerization technique (R. K. Dey, G. S. Tiwary, Tanushree Patnaik, Usha Jha) 15
Ethanol served as an ideal solvent for dissolving both the PAA and Shellac-PAA. In acetone the Shellac-PAA was found to be partially soluble. In acetone the Shellac-PAA was found to be partially soluble. Intrinsic viscosity in ethanol for Shellac-PAA was calculated to be 0.247 dL/g.
2.5 Modification of Natural Shellac using a diamine
(Jinwei Wang, Lei Chen, Yedong He) 2
Due to the growing concern on the environment and health made it necessary and extremely urgent and valuable to develop environmental friendly coatings. Many commonly used coatings such as polyurethane, epoxy etc. were produced as waterborne coating, solvent-free paint, and radiation curing paints, etc. so that their injury to people and environment could be reduced as little as possible. Shellac is one such environmental friendly coating based on natural product. Shellac was prepared by using aliphatic diamine as a crosslinking agent and ethanol as a solvent. Experimental procedure as reported in (Jinwei Wang, Lei Chen, Yedong He) 2 is that 30 ml purified shellac in ethanol solution (10%wt.) was poured into a flask with mechanic siring. When the solution temperature reached 50 â-¦C, 1,3-propanediamine (5%wt.of shellac) was dropped into the flask in 30 min followed by continuously stirring for another 1 h. The modified shellac could be obtained as a yellowbrown solution which could be used directly or deep brown powder by evaporating the ethanol at 45 â-¦C under vacuum. Natural Shellac was modified with 1,3-propanediamine by using Ethyl alcohol functioning as a solvent. When compared this modified shellac to the usual shellac, it was observed that the anticorrosive performances of the modified shellac on copper was greatly improved. These betterments were said to have occurred due to the establishment of network structure and the origination of hydrophobic propane chain in the shellac structure, and hence reducing the penetration speed of ionics and water onto the surface of copper. Other organic diamine such as, ethylene diamine, 1,6-hexamethylenediamine, etc. can also be used for similar modification. Depending on the chain lengths of these aliphatic groups, we might have different effect on the protective performance of the modified shellac.
3. Conclusion
Thus, considering the various limitations of Shellac like pH sensitivity, limited solubility, less stability, etc we can employ the modifications described in the above section. Modifications can include blending with another polymer to form a biocompatible polymer, or crosslinking. It can also be done by converting Shellac to some other material, as in this case, Maleated Shellac. Such modifications are of great importance which will result in better heat resistance, gloss, hardness, water resistance, etc. and overcoming of limitations of Shellac, which is a vital component in the field of biopolymer.
References
- Sitthiphong Soradech, Jurairat Nunthanid, Sontaya Limmatvapirat, Manee Luangtana-anan (2011) “An approach for the enhancement of the mechanical properties and film coating efficiency of shellac by the formation of composite films based on shellac and gelatin” Journal of Food Engineering 108 (2012) 94–102
- Jinwei Wang , Lei Chen, Yedong He (2008) “Preparation of environmental friendly coatings based on natural shellac modified by diamine and its applications for copper protection” Progress in Organic Coatings 62 (2008) 307–312
- Jennifer T. Otto, David L. Trumbo (2010) “A shellac derivative in thermoset coatings” J. Coat. Technol. Res., 7 (4) 525–527, 2010
- Melissa Gurgel Adeodato Vieira, Mariana Altenhofen da Silva, Lucielen Oliveira dos Santos, Marisa Masumi Beppu (2010) “Natural-based plasticizers and biopolymer films: A review” European Polymer Journal 47 (2011) 254–263
- Eva-Lena Hult, Marco Iotti, Marianne Lenes (2010) “Efficient approach to high barrier packaging using microfibrillar cellulose and shellac” Cellulose (2010) 17:575–586
- Syverud K, Stenius P (2009) Strength and barrier properties of microfibrillar cellulose (MFC) films. Cellulose 16(1):75–85
- Phan The D, Debeaufort F, Luu D, Voilley A (2008) Moisture barrier, wetting and mechanical properties of shellac/agar or shellac/cassava starch bilayer bio-membrane for food applications. J Membr Sci 325:277–283
- Sonja Berg, Manuela Bretz, Eva Maria Hubbermann, Karin Schwarz (2011) “Influence of different pectins on powder characteristics of microencapsulated anthocyanins and their impact on drug retention of shellac coated granulate” Journal of Food Engineering 108 (2012) 158–165
- Krause, K.P., Müller, R.H., (2001) “Production of aqueous shellac dispersions by high pressure homogenisation”. International Journal of Pharmaceutics 223 (1–2), 89–92
- S. K. Sharma, S. K. Shukla, D. N. Vaid (1983) “Shellac-Structure, Characteristics & Modification” Def Sci J, yo] 33, No.3, July 1983, pp 261-271
- Clemens, RJ, Rector, FD (1989) ‘‘Synthesis of Acetoacetyl—Functional Resins.’’ J. Coat. Technol., 61 770
- Hasmukh S. Patel and Sumeet J. Patel (2010) “Novel Surface Coating System Based on Maleated Shellac” E-Journal of Chemistry 2010, 7(S1), S55-S60
- D. N. Goswami and S. Kumar (1984) “Study on the Curing of Shellac with Epoxy Resins by Dielectric Measurements” Die Angewandte Makromolekulare Chemie 126 (1984) 145 – 152 (Nr. 1992)
- S. K. M. Tripathi, S. Kumar, G. S. Misra, Indian J. Technol. 4 (1966) 15
- R. K. Dey, G. S. Tiwary, Tanushree Patnaik, Usha Jha (2012) “Shellac-Polyamidoamine: Design of a New Polymeric Carrier Material for Controlled Release Application” Journal of Applied Polymer Science, Vol. 125, 2626–2635 (2012)
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


