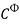Removal of TBP From Aqueous Phase
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1973 words | ✅ Published: 29 Jan 2018 |
ABSTRACT
PUREX process involves the use of 30% TBP in Dodecane to extract the fissile materials. However, due to mutual solubility some amount of TBP gets transferred into the aqueous phase. This transferred TBP leads to many environmental problems. Removal of this TBP from aqueous phase is of prime concern which can be done by contacting it with an organic diluent. UNIFAC and Uniquac methods have been used to describe Liquid Liquid equilibrium (LLE) in TBP-Diluent-HNO3 system. Uniquac and UNIFAC Group interaction parameters have been founded to fit the experimental data. Various metal nitrates are also present in the organic phase. These metal nitrates affect the solubility of TBP in aqueous phase. Metal nitrates like Sodium and Calcium nitrate have also been incorporated in model to find out interaction parameters in the presence of metal nitrates like Sodium and Calcium. The obtained parameters will be useful in predicting LLE for the above system and will aid in safe disposal of nuclear waste.
INTRODUCTION
Reprocessing of the used nuclear fuel has always been carried out to reduce the volume of high level radioactive waste and also for their safe disposal. The plutonium uranium extraction (PUREX) process is widely used for reprocessing. This process uses 30% Tri-n-butyl phosphate (TBP) in an inert paraffinic diluent for the separation of uranium and plutonium from the aqueous phase containing nitric acid. Mutual solubility of aqueous and the organic phase leads to the transfer of certain finite amount of TBP in aqueous phase. This transferred TBP decomposes very slowly in the presence of water and nitric acid by hydrolysis to lower organo-phosphate acids at normal operating temperatures leading to many environmental problems. Removal of such dissolved TBP is of direct interest in reprocessing processes as this would enable manifold evaporation of aqueous stream without harming the environment. Various metal elements are also present in the highly radioactive liquid waste solution in nitrate form. The salting out of TBP takes place in the presence of these inorganic nitrates in the aqueous phase. Studies in the presence of various metal nitrates will aid in efficient removal of TBP from aqueous phase.
In order to predict the extent to which TBP could be removed from aqueous phase, a model must be developed to predict the phase behaviour. Such models can be used for designing remediation projects. Estimation of activity coefficients of the mixtures is important for predicting the phase behaviour In order to predict the extent of mass transfer, chemical compositions of the two-phase system at equilibrium needs be predicted first.
Investigators have used various models to predict the LLE. Cheng et al. have calculated the thermodynamic equilibrium constant for the system HNO3-TBP-n-C7H16.The activity coefficient of nitric acid was calculated using Pitzer’s equation and those of the components in organic phase was derived from experimental data. Ding et al. have calculated the activity coefficient for 20 binary and 7 ternary systems composed of nC6H14, nC7H16, nC8H18, C6H6, cy-C6H6, CCl4, CHCl3, (C4H9)3PO4 and UO2(NO3)2.2((C4H9)3PO4) using head-space gas chromatography. The results are compared with Scatchard-Hilderbald, NRTL and UNIQUAC models. Li et al. has calculated the Vapor-Liquid and Vapor-Liquid-Liquid equilibria of 19 tributyl phosphate systems. Aqueous phase activity coefficients are calculated using Pitzer’s equation. UNIFAC method is used for correlating and predicting the data in organic phase. All the above authors have done work on the systems in the absence of metal nitrates. Interaction parameters for systems in the presence of metal nitrates have not been reported yet.
The objective of the present work to find the UNIFAC and Uniquac group interaction parameters regressed for the experimental data for three systems. These systems comprise of TBP-diluent-HNO3, TBP-diluent-HNO3-NaNO3, TBP-diluent-HNO3-Ca(NO3)2. These parameters will aid in predicting the equilibrium and calculating the number of stages for designing the equipment to remove dissolved TBP.
LIQUID LIQUID EXTRACTION EQUILIBRIA
The organic phase consists of (1) diluent (NPH), (2) TBP, (3) TBP.HNO3
The dissolution of TBP in NPH and HNO3 can be represented by eq 1and eq 2.
 xTBPorg (TBP)x org(1)
xTBPorg (TBP)x org(1)
 TBPorg + H+aq + NO3-aq HNO3.TBPorg (2)
TBPorg + H+aq + NO3-aq HNO3.TBPorg (2)
where the subscripts aq and org denote the species in the aqueous and organic phase.
The thermodynamic equilibrium constant for reaction (2) can be calculated as
a HNO3.TBP(org) a 3 (3)

 K ==
K ==
a H+(aq) a NO3-(aq) a TBP(org)a2±HNO3 a 2
x3 γ3
 K = (4)
K = (4)
m±2 γ±2 x2 γ2
where a is the thermodynamic activity, x is the mole fraction of the component in the organic phase and γ is the corresponding activity coefficient. m± is the mean molality concentration of electrolyte in the aqueous phase and γ± is the mean ionic activity coefficient of corresponding electrolytes. Similar procedure as described by Chen et al has been used to predict the LLE with one extraction reaction.
Mean ionic activity coefficient of electrolytes
Pitzer equation is used to calculate the mean ionic coefficient of HNO3 in all the cases and of Ca(NO3)2, NaNO3 in the presence of metal nitrates.
lnγ± = – AФ + m
+ m (5)
(5)
where b=1.2, α=2, Aɸ = 0.391. I is the ionic strength of solution. The Pitzer parameters for HNO3, NaNO3 and Ca(NO3)2 are listed in Table 1.
Activity Coefficients of components in organic phase can be estimated using UNIFAC and Uniquac equation.
UNIFAC EQUATION
In a multi-component mixture, the UNIFAC equation for the activity coefficient of component i is given by Equation 3.1.
ln γi = ln γiC +ln γiR (6)
The combinatorial part of the UNIFAC model considers the shape and the size of the molecules in the mixture.
ln γiC = 1 – Ji + ln Ji – 5qi( ln (ɸi /Θi )+ 1 – (ɸi /Θi ) ) (7)
where, Ji = ( ɸi /xi)
The molecule volume fraction ɸi , and the molecule surface area fraction Θi , are given by
respectively,
ɸi = xi*ri/∑j xj*rj and Θi = xi*qi/∑j xj*qj (8)
In Equations (3.3), relative molecular volume rs, and relative molecule surface area q, are given by
ri = ∑k νk(i)* Rk and qi = ∑k νk(i)* Qk (9)
The quantity vk is the number of subgroups of type k in a molecule of species i. ri is the relative molecular volume and qi is the relative molecular surface area. Group parameters Rk and Qk are reported by Fredenslund et al.
The residual part of the activity coefficient is given by Equation 10.
ln γiR = ∑k νk(i )[ ln Гk – ln Гk(i) ] (10)
where,
k denotes each group in the mixture
νk(i ) is the number of groups of type k in molecule i
Гk is the group residual activity coefficient
Гk(i) is the residual activity coefficient of group k in a reference solution containing only molecules of type i.
The group residual activity coefficient is found by Equation 11.
ln Гk = Qk [ 1- ln(∑m Θm ψmk) – ∑m (Θm ψkm /∑n Θn ψnm ) ] (11)
where,
Qk is a group surface area parameter
Θm is the area fraction of group m
ψmn is the group energy of interaction parameter
ψmn = exp(-amn + bmn/T) (12)
Where,
amn and bmn is the group-interaction parameter.
T is the temperature
Group assignment as proposed by Chen et al. has been followed. C7H16, TBP and TBP.HNO3 has been broken down into groups CH3, CH2, (CH2O)3PO, HNO3.(CH2O)3PO. Group volume and surface parameters for above groups have been reported by Cheng et al.
The UNIQUAC model thus consists of two terms: a combinatorial or entropic term, a residual or enthalpic term.
The combinatorial and the residual terms are identical to the terms used in the traditional UNIQUAC equation. The combinatorial, entropic term is
ln γiC = ln(ɸi/xi) +1 – (ɸi/xi) – (z/2)*qi [ ln(ɸi/Θi) + 1- (ɸi/Θi) ] (13) z = 10 is the co-ordination number. xi is the mole fraction, ɸi is the volume fraction, Θi and is the surface area fraction of component i.
ɸi = xi*ri/∑j xj*rj and Θi = xi*qi/∑j xj*qj (14)
ri and qi are volume and surface area parameters for component i. The residual, enthalpic term is
ln γiR = qi [ 1 – ln( ∑k Θkψki) – ∑k Θkψik/(∑l Θlψlk) (15)
The parameter ψki is given by
ψki = exp(-uki-uii/T) (16)
uki and uii are interaction energy parameters.
Uniquac r and q parameters for C7H16, TBP and TBP.HNO3 have been reported by Li et al.
RESULTS AND DISCUSSION
EQUILIBRIUM PREDICTION.
The following equations can be used to predict equilibrium
x1 + x2 + x3 = 1 (17)
x20 = (x2 + x3)/( x1 + x2 + x3) (18)
x3 γ3
 K = (19)
K = (19)
m±2 γ±2 x2 γ2
γ2 and γ3 values have been calculated using UNIFAC and Uniquac equation. Knowing equilibrium constant K, m± calculated mole fractions can be found out., The group interaction parameters are regressed by Least Square Technique to minimize the error between experimental and calculated mole fraction values. The obtained regressed group interaction values in the absence of metal nitrates, in the presence of NaNO3 and Ca(NO3)2 using Uniquac and UNIFAC have been listed in Table. The standard absolute deviation of components in the organic phase is listed in table. The experimental and calculated values of mole fractions have been reported graphically in fig
CONCLUSION
The experimental mole fraction data were correlated using UNIFAC and Uniquac model. The Uniquac and UNIFAC group interaction parameters are capable of predicting mole fraction for TBP-Diluent-HNO3 in the absence and presence of metal nitrates. Thus these can be effectively used to predict the equilibrium for the removal of dissolved TBP in Nuclear engineering. UNIFAC gives a better prediction as compared to Uniquac in all the cases
LIST OF TABLES
- Pitzer parameters for calculation of mean ionic activity coefficient
- Uniquac Group interaction parameters in the absence of metal nitrates
- Unifac Group interaction parameters in the absence of metal nitrates
- Uniquac Group interaction parameters in the presence of Sodium metal nitrate
- Unifac Group interaction parameters in the presence of sodium nitrate
- Uniquac Group interaction parameters in the presence of calcium metal nitrate
- Unifac Group interaction parameters in the presence of calcium nitrate
- Standard absolute deviations between predicted and experimental mole fraction of extracted complexes
Table 1. Pitzer parameters for calculation of mean ionic activity coefficient
|
Components |
βo |
βo |
|
|
HNO3 |
0.1119 |
0.3206 |
0.001 |
|
NaNO3 |
0.0068 |
0.1783 |
-0.0007 |
|
Ca(NO3) |
0.2108 |
1.409 |
-0.02014 |
Table 2. Uniquac Group interaction parameters in the absence of metal nitrates
|
a (m,n) |
NPH |
TBP |
HNO3.TBP |
|
NPH |
0 |
2.05741 |
0.679624 |
|
TBP |
-3.04549 |
0 |
-1.1645 |
|
HNO3.TBP |
-1.03328 |
1.143979 |
0 |
|
b (m,n) |
NPH |
TBP |
HNO3.TBP |
|
NPH |
0 |
1.007842 |
0.999125 |
|
TBP |
0.983532 |
0 |
1.095824 |
|
HNO3.TBP |
0.993173 |
1.019649 |
0 |
Table 3. Unifac Group interaction parameters in the absence of metal nitrates
|
a (m,n) |
CH2 |
CH3 |
(CH2O)3PO |
HNO3(CH2O)3PO |
|
CH2 |
0 |
0 |
2.56180892 |
-48.1996 |
|
CH3 |
0 |
0 |
2.56180892 |
-48.1996 |
|
(CH2O)3PO |
-5.15816 |
-5.15816 |
0 |
-6.13375 |
|
HNO3(CH2O)3PO |
-2.57271 |
-2.57271 |
34.781685 |
0 |
|
b (m,n) |
CH2 |
CH3 |
(CH2O)3PO |
HNO3(CH2O)3PO |
|
CH2 |
0 |
0 |
0.997309 |
1.126187 |
|
CH3 |
0 |
0 |
0.997309 |
1.126187 |
|
(CH2O)3PO |
1.016819 |
1.016819 |
0 |
1.131422 |
|
HNO3(CH2O)3PO |
1.015345 |
1.015345 |
0.890301 |
0 |
Table 4. Uniquac Group interaction parameters in the presence of Sodium metal nitrate
|
a (m,n) |
NPH |
TBP |
HNO3.TBP |
|
NPH |
0 |
-1.82623 |
6.863001 |
|
TBP |
0.87821 |
0 |
0.552002 |
|
HNO3.TBP |
1.380676 |
2.272663 |
0 |
|
b (m,n) |
NPH |
TBP |
HNO3.TBP |
|
NPH |
0 |
0.990771 |
1.019671 |
|
TBP |
0.999592 |
0 |
0.998537 |
|
HNO3.TBP |
1.001281 |
1.004269 |
0 |
Table 5. Unifac Group interaction parameters in the presence of sodium nitrate
|
a (m,n) |
CH2 |
CH3 |
(CH2O)3PO |
HNO3(CH2O)3PO |
|
CH2 |
0 |
0 |
0.742770 |
-0.6378 |
|
CH3 |
0 |
0 |
0.742770 |
-0.6378 |
|
(CH2O)3PO |
1.096426 |
1.096426 |
0 |
-0.373895 |
|
HNO3(CH2O)3PO |
0.748111 |
0.748111 |
-0.20966206 |
0 |
|
b (m,n) |
CH2 |
CH3 |
(CH2O)3PO |
HNO3(CH2O)3PO |
|
CH2 |
0 |
0 |
1.000835 |
1.005434 |
|
CH3 |
0 |
0 |
1.000833 |
1.005434 |
|
(CH2O)3PO |
0.99968 |
0.999688 |
0 |
1.004477 |
|
HNO3(CH2O)3PO |
1.000817 |
1.000818 |
1.003927 |
0 |
Table 6. Uniquac Group interaction parameters in the presence of Calcium metal nitrate
|
a (m,n) |
NPH |
TBP |
HNO3.TBP |
|
NPH |
0 |
0.3249 |
-0.4026 |
|
TBP |
0.95221 |
0 |
-1.40706 |
|
HNO3.TBP |
1.168545 |
1.39797 |
0 |
|
b (m,n) |
NPH |
TBP |
HNO3.TBP |
|
NPH |
0 |
0.997878 |
0.99529 |
|
TBP |
0.999836 |
0 |
0.99200 |
|
HNO3.TBP |
1.00058 |
1.000138 |
0 |
Table 7. Unifac Group interaction parameters in the presence of calcium nitrate
|
a (m,n) |
CH2 |
CH3 |
(CH2O)3PO |
HNO3(CH2O)3PO |
|
CH2 |
0 |
0 |
5.9429 |
2.14979 |
|
CH3 |
0 |
0 |
5.9429 |
2.14979 |
|
(CH2O)3PO |
2.6932 |
2.6932 |
0 |
-2.59369 |
|
HNO3(CH2O)3PO |
3.8889 |
3.8889 |
3.8740 |
0 |
|
b (m,n) |
CH2 |
CH3 |
(CH2O)3PO |
HNO3(CH2O)3PO |
|
CH2 |
0 |
0 |
0.9839 |
0.99622 |
|
CH3 |
0 |
0 |
0.9839 |
0.99622 |
|
(CH2O)3PO |
0.99447 |
0.99447 |
0 |
1.011186 |
|
HNO3(CH2O)3PO |
0.990612 |
0.990612 |
0.990633 |
0 |
Table 8. Standard absolute deviations between predicted and experimental mole fraction of extracted complexes.
|
System |
Δ x (Uniquac) |
Δ x (Unifac) |
|
HNO3/TBP/Diluent |
0.016 |
0.0051 |
|
HNO3/TBP/Diluent/NaNO3 |
0.0435 |
0.0429 |
|
HNO3/TBP/Diluent/Ca(NO3)2 |
0.015 |
0.0051 |
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal