Procedure for Extracting Paracetamol
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 3522 words | ✅ Published: 23 Sep 2019 |
Assignment title
Preparing Aspirin
You are a research and development technician, working for a company trying to optimise solvent extraction for one of its preparative processes.
Complete the procedure to extract aspirin and write a lab report.
Tasks
Task 3.1 – Production (Part of P1)
Follow the procedure to prepare a sample of aspirin.
Make sure you clearly record all the information and data that the task sheet asks for.
Your teacher will complete the observation record found at the bottom of the procedure worksheet.
Task 3.2 – Calculations (Part of P2)
Create a report on the yield of your prepared substance. Follow the method on the worksheet to calculate the percentage yield of the reaction, the purity of the aspirin and the atom economy of the reaction.
Task 3.3 – Scientific Principles (Part of M1)
Describe the scientific principles behind the key steps in the preparation of aspirin (produced in Task 3.1). You can include diagrams to support explanations.
Task 3.4 – Problems (Part of M2)
Describe the main problems you encountered whilst following the procedures from Task 3.1. It is important that this isn’t just a list of things that went wrong, give details about why it was a problem and how it arose. You can also rank the problems in terms of easiest to put right to most difficult or from having the biggest to the least effect on the end product.
Task 3.5 – Changing the Yield (Part of D1)
Create a report explaining how the yield, purity and atom economy of the production of aspirin could be changed.
Give straight forward changes you could make to the method to alter (increased or decreased) the:
- percentage yield of aspirin produced
- purity of the aspirin produced
- The atom economy of the reaction.
Explain how and why your changes would have an effect on the yield, purity and atom economy.
You could complete this in the same report as Task 3.4.
Task 3.6 – Sources of Error (Part of M5)
Create a report identifying the sources of error and uncertainty in your measurements taken in task 1.1.
The sources need to be specific and realistic reflecting the method and equipment they used. General statements like ‘error reading the equipment’ are not detailed enough to be awarded M5.
Task 3.7 – Evaluation (Part of D3)
Create a report evaluating the reliability of your measurements throughout the procedure. How reliable are your values? Why do you think that?
You should compare your values to:
- Those obtained from other students in the class; calculate an average value and the difference between your value and the average.
- Those obtained by the teacher who will also carry out the practical; calculate the difference between your value and the teacher’s and this as a percentage of the teacher’s value.
You could complete this in the same report as Task 3.6
INTRODUCTION
I’m a research and development technician and I’m working for a company trying to optimise solvent extraction processes.
The produced acetic acid was removed by filtration of an acetic acid-water solution to get crystals of aspirin. Traces of salicylic acid residues were removed by recrystallization from an ethanol/water solvent pair.
In this experiment, aspirin can be synthesised and purified. In addition, the purity of the product will be determined by qualitative analysis measurement of its melting point range.
RISK ASSESSMENT
Methyl salicylate and salicylic acid are flammable and harmful if swallowed. Eye goggles must be worn. Ethanoic anhydride is corrosive and causes severe burns. Care must be taken when carrying out the procedure and can become boiling. Sulfuric acid is also corrosive and protective must be gloves worn.
EQUIPMENT
Beaker
Distilled water
Tripod
Gauze
Round-bottomed flask
Condenser
5.0g of salicylic acid
5.0g of sulfuric acid
5.0g of ethanoic anhydride
5.0 g of phosphoric acid
Weight scale
Reflux
Ice bath
Fume cupboard
Boil water
Stirling rod
Cold water
Buchner apparatus
Filter paper
Anti-bumping granules
Thermometer
METHODS
1. Place a 250 cm3 beaker, half-filled with water, on top of a tripod and gauze.
2. Clamp a 50 cm3 round-bottomed flask so that it is partially submerged in the beaker of water, and fit a reflux condenser to it vertically. Start to run water through the condenser.
3. Add two anti-bumping granules to the flask. Then weigh out 5.0 g of salicylic acid, 5.0 g of ethanoic anhydride, and 5.0 g of phosphoric acid into separate weighing bottles and add them, separately, into the flask through a funnel placed on top of the condenser.
4. Heat the water in the beaker and reflux the mixture for 30 minutes. While this is happening, pour 150 cm3 of distilled water into a 250 cm3 beaker and place it in an ice bath.
5. Allow the reaction mixture to cool down for a few minutes, then dismantle the condenser and pour the reaction mixture into the cold water. Stir for a few minutes and allow the mixture to settle.
6. Filter the mixture through Buchner apparatus. Wash any residue remaining in the beaker into the funnel with more distilled water.
7. Remove the filter paper with the solid residue on it and scrape it into another beaker.
8. Recrystallise the aspirin from the minimum quantity of boiling water.
9. Once the product is dry, transfer it into a weighing bottle and weigh it.
RESULTS AND CALCULATIONS
1st experiment:
0.067/180 = 0.000372 mol
Expected no. of moles = 3.073/180 = 0.0171mol
Percentage yield = actual yield = 0.000372 x 100 = 2%
Theoretical yield 0.0171
1st experiment:
0.067/180 = 0.000372 mol
Expected no. of moles = 3.073/180 = 0.0171mol
Percentage yield = actual yield = 0.000372 x 100 = 2%
Theoretical yield 0.0171
Average percentage yield
1 mole of aspirin is 180g
Molar mass 180g/mol
Average percent yield
Percentage yield = 42%
2nd experiment:
1.799/180 = 0.009994 mol
Expected no. of moles = 3.054/180 = 0.01696 mol
Percentage yield = actual yield = 0.009994 x 100 = 59%
Theoretical yield
3rd experiment:
2.032/180 = 0.01128 mol
Expected no. of moles = 3.074/180 = 0.01707 mol
Percentage yield = actual yield = 0.01128 x100 = 66%
Theoretical yield 0.01707
3rd experiment:
2.032/180 = 0.01128 mol
Expected no. of moles = 3.074/180 = 0.01707 mol
Percentage yield = actual yield = 0.01128 x100 = 66%
Theoretical yield 0.01707
SCIENTIFIC PRICIPLES (Part of M1)
Salicylic acid is a parent compound of aspirin. In this experiment, is all about synthesise aspirin, purify it, and find the percent yield. Qualitative analysis and measurement of its melting point range will confirm the purity of the product.
Acetylsalicylic acid was found to have the medicinal properties. The acetyl group masks the acidity of the drug during ingestion. Upon entering the small intestine, it converts to salicylic acid, where it can enter the bloodstream and relieve pain. But it also has flaws, it can cause stomach irritation in some people. Aspirin is an important analgesic, so increase it purity is essential.
In this reaction, an excess of acetic anhydride was (added speeds up the reaction) to the measured mass of salicylic acid in the presence of the catalyst sulfuric acid. The mixture is heated to form acetylsalicylic acid and acetic acid. After the reaction occurred, water was added to destroy excess acetic anhydride and crystallize the product. Aspirin was then collected, purified by recrystallization.
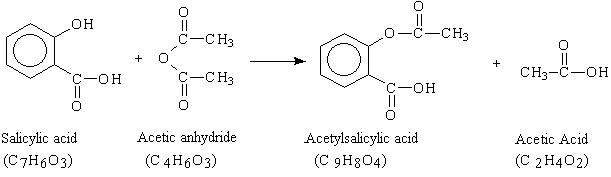
Reaction mechanism
The above is the reaction of aspirin formations. The organic synthesis is an esterification reaction between a compound containing an OH group (ester) and an acid. An ester is an organic acid, so hydroxyl group is substituted. The H from the OH group is replaced by a carboxy carbon C=O group.
Esterification is an acid catalyzed reaction of a carboxyl group (-COOH) with an alcohol or a -OH group of a phenol to form a carboxylic acid ester. The reaction does require a catalyst such as concentrated H2SO4.
In this reaction, an excess of acetic anhydride (C 4 H 6O 3 ) was added to the measured mass of salicylic acid (C 7 H 6 O 3 ) in the presence of the catalyst sulfuric acid (H 2 SO 4 ). The mixture is heated to form the acetylsalicylic acid and acetic acid.
What is the purpose of recrystallizing the aspirin?
The crude aspirin contains salicylic acid as its principal impurity, and recrystallization gives us a convenient purification method.
Recrystallization refers to fractional crystallisation, and it is a scientific method to purify impure compounds in solvents to get purity products. The purification method is based on the principle that: most solid solubility increases as temperature increases. This means as the temperature rises, the dissolved mass dissolved in the also solvent increases. So in another word, during recrystallization, an impure solid compound is dissolved in a hot liquid until the solution is saturated, and then the liquid is allowed to cool.
Why is aspirin insoluble in cold water?
Aspirin is only slightly soluble in water is because aspirin contains polar functional groups that form hydrogen bonds with polar water molecules. So excess reactants could be washed away with cold water.
PROBLEMS AND CHANGING THE YIELD (Part of M2 and D1)
Some common problems, such as equipment calibrating and maintenance. They may not sound like a big deal, but if used incorrectly, it will still affect and contaminate the result. For example, if the reflux water-condenser has a stiff, blocked or leaking plug valve, the experiment will not succeed.


I’ve also noticed that the percent yield of the first experiment was 2%. In comparison, there was a slight error. Maybe the sample is not properly weighed or another problem have occurred. This may be due to processing problems such as suction filtration or drying. Because Buchner wasn’t working properly, so during the particle we wait till sample to dry out, the result may not be that satisfaction.
The main problem I encountered was when filter paper was unable to tolerate a vacuum, so most mixture is most likely to spill out or collapse. For the improvement, try a sintered glass funnel – they have a porous glass membrane (available in different grades) instead of a large hole in the Buchner funnel, so no filter paper is needed and is more robust and should not collapse under vacuum.
Some part of the advance filter unit will have a pressure/vacuum meter, also try to use some filter solvent to wet the filter to ensure the paper sticks to the filter funnel. When is paper is properly sticking to the filter funnels, then start with a very low vacuum and slowly add the mixture to start the filtration. Once you have built a few millimeters, then increase vacuum slightly.
Sometimes, the compound does not recrystallize completely, this is due two main reasons: Used too much solvent. It must fully evaporate or it will form a supersaturated solution. Recrystallization is generally not considered a separation technique; rather, it is a purification technique in which a small number of impurities are removed from the compound. However, if the solubility properties of the two compounds are sufficiently different, recrystallization can be used to separate them even if they are present in almost equal amounts. The recrystallization effect is best when most of the impurities have been removed by another method such as extraction or column chromatography.


Purity can be affected by several variables such as temperature and reactant concentration solvent. The atomic economy is also related to yield and purity. The yield and rate of the Chemical reaction also depending on some conditions such as temperature and pressure. Since water is also a product, make sure that there is no water in the bottle before the reaction begins, as the water will deviate from the production of the products.
Chances to achieves 100% of the yield or pure products are small, but there is some better method could approach in order to increase the percentage yield or purity.
Generally, the percent yield is understandably less than 100% due to incomplete reactions and the reactants may not complete when converted to product. If a reverse reaction occurs, the final state contains reactants and products in a chemical equilibrium state. However, if the measured product of the reaction contains impurities which cause the actual mass to be greater than the purity of the actual product, the yield may actually exceed 100%.
Aspirin is made of acetylsalicylic acid and naturally decomposes into salicylic acid and acetic acid. In fact, if you have old aspirin try to smell it, it will smell like vinegar it means is old and will cause effects on purity. Typically, the moister environment is preferred when you store it in. In most case, the main important responsible for achieving good crystallization it depends on, how well acetic acid has been a pollutant. If you do not solve all the problems, it will affect the slow hydrolysis of acetyl groups, in addition to the acetic acid flavor.
Any purity techniques can affect aspirin production and purity. The process such as mixing, heating, cooling, filtration, washing, and drying, all that can affect aspirin production and purity. Because of chemical reactions, aspirin produced most of the time may be impure already, therefore, aspirin must go through another process also known as, re-crystallization. Even better recrystallization should be carried out several times until there is no impurity in aspirin. Some of the other factors that affect purity, such as, impurities that may be present in the glassware that already used, also, depending on the number of chemicals used.
There is a better method when preparation of aspirin during the recrystallization process. It is improved by: Hence the loss of aspirin and the reduction in aspirin quality after each recrystallization limit the number of crystals to obtain from the purist aspirin. Therefore, if I double the mass of the reactants, the quality of the produced aspirin will likely to increase and the number of recrystallizations will double.
In conclusions, organic synthesis must be practiced perfectly to achieve high or improve percentage yield or purity. The simplest effective things to do to get better yield or purity is be careful while weighing/ measuring starting materials (Purity should be high when is not contaminate) and while ensure mixing is well mixed as well as be cautious in transferring and drying. Furthermore, let the solution cool as slowly as possible and this will maximize the crystal size, keep the relative supersaturation of the solution low, which will increase crystal size and increase purity.
We can also use different aspirin purification methods, such as thin layer chromatography, which is a sensitive and fast method for detecting impurities in organic products (aspirin). With this procedure is also tell me how close my aspirin is from pure aspirin and how close it is to salicylic acid.
SOURCES OF ERROR AND EVAULATION (Part of M5 and D3)
During the dipping, if the burette reading is not read at the eye level, it may cause a parallax error. Therefore, when reading from the burette, the level of the eye should be the same as the level of the meniscus. Also when we analysis different recrystallized samples, the colour of the indicator changes from pale pink to transparent, making it difficult to judge when the reaction is going to complete.
The random error could also occur, for instance, the apparatus I used also had uncertainties like the measuring flask has an uncertainty of ±0.5cm3. This will cause my results to go wrong. Use equipment with less error to reduce equipment errors Is always a better choice. For example, I could replace a burette with an error of ±0.1 cm3 with a burette with an error of ±0.05 cm3.
I also noticed when I mixed the salicylic acid and the ethanolic anhydride solution in concentrated sulfuric acid, it was difficult to form a crystalline paste of aspirin by stirring the flask. Try to use a magnetic stirrer, it stirred evenly, in this way, all chemicals should be mixed properly.

This will be more effective in producing mush. Actually, many aspirates can be lost during recrystallization. When I used filter paper, I cut it to the same size as the Hirsh funnel. Instead, I can use a larger filter paper in the funnel so that impurities don’t leak out of the edge of the filter, and I can get a cleaner sample of aspirin.
When aspirin is placed in an evaporating dish and dried overnight, many contaminants could be mixed into the aspirin. In reality, If this is not taken into account in the pharmaceutical industry, this can lead to serious health problems. Therefore, when placed overnight to dry, it should cover up properly.
Spectrophotometers are also another reliable and economical way to maintain consistent aspirin purity throughout the manufacturing process. The purity and amount of acetylsalicylic acid in aspirin can be measured using a visible spectrophotometer. This is because of visible violet colour reaction. This also explains when the iron is added to aspirin, it produces a purple salicylic blue iron complex.
Since purple has the shortest wavelength (400-420 nm), so it can be easily measured by the visible spectrophotometer. It using a range of different concentrations of aspirin, this will at the different calibration curve and can be constructed as a spectrophotometer will measure each solution baseline for the amount of aspirin in a given aspirin product.
Average percentage yield
1st experiment:
0.067/180 = 0.000372 mol
Expected no. of moles = 3.073/180 = 0.0171mol
Percentage yield = actual yield = 0.000372 x 100 = 2%
Theoretical yield 0.0171
1 mole of aspirin is 180g
Molar mass 180g/mol
Average percent yield
Percentage yield = 42%
2nd experiment:
1.799/180 = 0.009994 mol
Expected no. of moles = 3.054/180 = 0.01696 mol
Percentage yield = actual yield = 0.009994 x 100 = 59%
Theoretical yield
3rd experiment:
2.032/180 = 0.01128 mol
Expected no. of moles = 3.074/180 = 0.01707 mol
Percentage yield = actual yield = 0.01128 x100 = 66%
Theoretical yield 0.01707
3rd experiment:
2.032/180 = 0.01128 mol
Expected no. of moles = 3.074/180 = 0.01707 mol
Percentage yield = actual yield = 0.01128 x100 = 66%
Theoretical yield 0.01707
Significant figures are an important part of scientific calculations, involving the accuracy and precision of numbers. It is important to estimate the uncertainty of the final result and significant figures usually become very important. The purpose of using significant figures is to track the quality (variability) of the measurements.
In science, all numbers are based upon measurements, since all measurements are uncertain, we must only use those numbers that are meaningful.
Try to add or subtraction, round by the least number of decimals. In this case, leading zeros are never considered as significant, however, any non-zero number are significant figures, for example, 0.0171mol have 3 SFs and we can fix it by round them up so it would be 0.017 moles (1,2,3,4 round off and 5,6,7,8,9 are round up).
Typical students will obtain 60-70% of yield. In conclusions, I assume that the final aspirin is impure and it is likely that most products are going to be impure, contaminations due to the recrystallization process.
However, further analysis is necessary to prove its exact purity. There may be problems that may affect the product, for example, when an initial amount of salicylic acid is dissolved in a solution of acetic anhydride and concentrated sulfuric acid, it may not completely be dissolved into the solution even when heated. This may have a slight effect on the overall yield of aspirin, as it is possible that not all salicylic acids are properly synthesized.
Another reason was not well mixed, although well-formed crystals are expected to be pure, each molecule or ion must be completely suitable for the crystal lattice as it leaves the solution.
Impurities would normally not fit as well in the lattice. However, there is a case where impurities are incorporated into the crystal lattice, thus lowering the purity level of the final crystal product.
Moreover, in some cases, the solvent can be incorporated into the crystal lattice to form a solvate. In addition, the solvent can get trap (liquid) within the formed crystal and this also known as inclusion compound (One chemical compound has a cavity into which “guest” compound which can be accommodated together).
REFERENCES AND BIBLIOGRAPHY
- Annets, Frances, et al. BTEC Level 3 National Applied Science Student Book, Pearson Education Limited, 2010. ProQuest Ebook Central,.
- Synthesis of aspirin worksheet.
- http://www.chem.latech.edu/~deddy/chem104/104Aspirin.htm
- https://thepoetrytrust.org/testing-the-purity-of-aspirin-biology-essay-essay
- https://sensing.konicaminolta.us/blog/how-spectrophotometers-can-ensure-the-purity-of-aspirin/
- http://chemistry.bd.psu.edu/jircitano/sigfigs.html
- https://freebooksummary.com/testing-the-purity-of-aspirin-biology-essay-essay
- http://www.a-levelchemistry.co.uk/unit-6.html
- http://www.magritek.com/wp-content/uploads/2015/03/Lab-Manual-Synthesis-of-Aspirin-web.pdf
- https://pdfs.semanticscholar.org/5e9a/f12eecb0d610768b5c45b4f249cab0fbe630.pdf
- https://www.chemguide.co.uk/organicprops/alcohols/esterification.html
- https://biocyclopedia.com/index/chem_lab_methods/problems_in_recrystallization.php
- https://www.jove.com/science-education/10184/purifying-compounds-by-recrystallization
- https://www.reddit.com/r/chemhelp/comments/pdmyb/help_recrystallization_sources_of_error/
- https://chem.libretexts.org/Textbook_Maps/General_Chemistry/Book%3A_Chem1_(Lower)/3%3A_Measuring_Matter/3.3%3A__Significant_figures_and_rounding_off
- https://www.nde-ed.org/GeneralResources/SigFigs/SigFigs.htm
- https://uk.answers.yahoo.com/question/index?qid=20120426233401AAw3wef
- https://study.com/academy/answer/how-to-calculate-the-theoretical-yield-of-aspirin.html
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


