Photocatalytic Degradation of Lignin
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 4759 words | ✅ Published: 18 May 2020 |
Current Energy Climate:
Despite the increased deliberation into sustainable methods and the substantial effort to mitigate emissions, analysis of the emission trends of the pharmaceutical industry have been documentedas being significantly more in comparison to the automotive industry. In an industry-specific comparative study, analysis showed that emissions were 55 % higher. Contributions from highest and lowest emitter varied by 5.5 times, highlighting the lack of environmental practices within the industry. Moreover, this study also highlighted that companies within the sector were able to reduce emissions without suffering financial loss and that only 20% of the companies investigated are expected to achieve the US target reductions of 25% (Figure 1). (Belkhir and Elmeligi, 2018).
Similarly, the European Union has also outlined an obligatory target whereby energy consumption by 2020 must comprise of renewable energy, where 10% is provided by biofuels (Zakzeski et al, 2010). The research into the employment of biomass such as lignin for manufacture of chemicals is likely to increase in importance with the increase in pressure to utilise sustainable production methods, coupled with the growing demand on fuel sources. It is evident that the targets outlined may elicit more severe regulations to be imposed on the environmental practices of the pharmaceutical industry, necessitating the research into alternative methods.

Fig. 1. Emission intensities for 15 pharmaceutical companies in 2015, shown with the projected levels in 2020 and 2025 and the associated targets (Belkhir, L., Elmeligi, A. 2018)
The relocation of manufacturing of pharmaceutical products from developed countries to developing countries has become increasingly standard. The growing demand in developing countries, and the ability to produce the same products more cost effectively has strongly impacted the drug development market. Figure 2 depicts the top emerging markets; Brazil, Russia, India and China, which were previously deemed as marginal producers. Due to the reduction of FDA approved clinical trials and increase in generic drugs, the competition within this sector has heightened. Therefore sustainable production, such as employing biomass as a method to produce chemicals in favour of green chemistry principles, has gained substantial interest. (Sławomir Dorock, 2014)
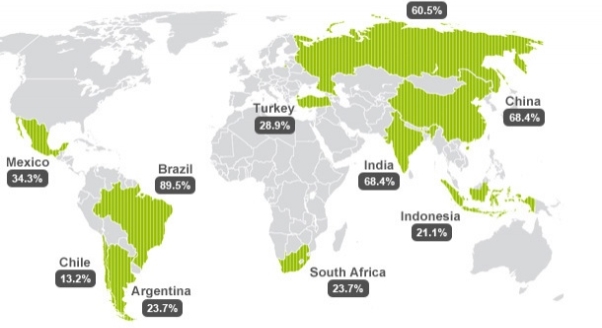
Figure 2. The rated top emerging pharmaceutical markets, 2012-2017. (Anna Räder 2012)
Biorefinery concept defined as “is the sustainable processing of biomass into a spectrum of marketable products and energy” (Cherubini, 2010). Whilst renewable energy is being used within a broad range of industries, there is potential for the pharmaceutical sector to use sustainable resources beyond just providing energy. Applications can be extended to ‘green’ chemical synthesis of pharmaceutically active compounds and incorporation into tablets for improved release rates of active materials. In order to explore the potential role of biomass in this sector however, it is vital that the associated technology and method of conversion is investigated. Identifying the major impediments and understanding the mechanism taking place may inform strategies that will be of considerable importance on a commercial scale and potentially for the generation of novel methods to produce pharmaceutical components.
Lignin availability:
The extensive availability of lignin can be attributed to both; the paper industry which produces several millions of tonnes of lignin preparations annually and soil matter, of which lignin is the largest contributor. According to a global industry growth analysis report published in March 2019, the global lignin market size was valued at 793.7 million US dollars in 2018 and is projected to expand at a compound annual growth rate of 1.9% from 2019 to 2025 (Grand View Research, 2018). Moreover, the structure of lignin which is mainly composed of aromatic moieties, ascertains how lignin derivatives is an attractive alternative for fine chemical synthesis and therefore sustainable production (Fransicso and Dobado, 2010). Due to the low monomer yield and the problems associated with the tendency of lignin to re-polymerise, studying the degradation pathway of lignin is crucial in order to overcome the shortcomings that prevent the commercialisation of lignin.
Lignin structure:
Lignin is amongst one of the primary components of plant biomass, comprising 15-30% wt. It contains several oxygen functional groups including hydroxyl, ether and ester groups which enables the formation of phenolic compounds via chemical degradation (Wahyudiono et al, 2008).
Currently, employing lignin in large scale processes is not readily applicable due to obscurity regarding its degradation pathway. The recalcitrant nature of lignin is owed to its amorphous and complex nature. Lignin is composed of hydroxycinnamyl alcohols, p-coumaryl, coniferyl, and sinapyl alcohol monolignols. These monolignols produce corresponding phenylpropanoid units when incorporated in the lignin polymer (Boerjan et al, 2003).
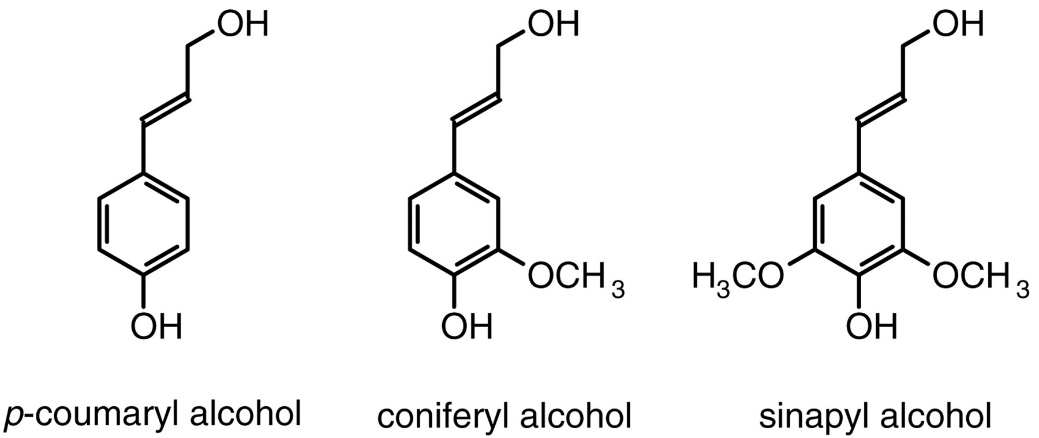
Figure 3. Structures of the three major monolignol subunits found in lignin. (Hatfield and Vermerris 2001).
Lignin into three major groups such as softwood lignin, hardwood lignin, and grass lignin due to differences in the chemical structure of monomer units and the linkages between monomer units. (Hatfield and Vermerris 2001). The complexity of the structure that has numerous possible combinations of monomers coupled with the variance of distribution of monomers, impedes understanding of reaction mechanism and consequently limits its usage.
Furthermore, understanding lignin solubilisation offers structural information that is since hindering valorisation and thus its industrial application. Lignin has poor solubility in a range of common organic solvents which limits how it can be studied in laboratory setting.
Phenylpropane monomers link together via C-O a and b ether bonds. Specifically, the B-O4 linkage constitutes 50% of all linkages found in lignin. Consequently, specific targeting and degradation of this bond has been studied intensely. The significance of this specific bond is further reinforced due to the fact that aromatic characteristics are preserved for further manipulation for fine chemical synthesis (Jia et al, 2010). Since photocatalytic process is non-specific, therefore selective targeting of this bond is still being investigated and consequently, derivation of phenolic compounds is one of numerous applications still in the process of development.
In order to produce aromatic compounds from lignin for bulk chemical production, cleavage of the β-O-4 ether bond is necessitated to produce phenolic compounds such as aldehydes, acids, and alcohols (Figure 6.) The main constraint that prevents upscaling this process is the propensity of lignin to re-polymerise, whereby unstable lignin fragments condense to form monomeric products instead (Toledano et al, 2014). This highlights that the principal issue with green chemical production techniques in comparison to conventional methods is that biological degradation of biomass is more complex and challenging than petroleum derived alternatives, that must be solved in order to become the main practice to produce chemicals.

Fig. 4. a) Conversion mechanism of poplar lignin to aromatic compounds – B-O4 unit displayed in blue. b) Structure of β-O-4 model compounds 1 and 2. (Rahimi et al, 2014)
Lignin – A sustainable platform for obtaining value added chemicals:
Obtaining value added chemicals from biomass is relevant to both scientific and industrial communities. The conversion of lignin to vanillin is applicable to the food, beverage and pharmaceutical industry. Vanillin production obtained from lignin amounts to 15 % annually, thus representing one of the only phenolic compounds, manufactured successfully on an industrial scale from biomass. Furthermore, the advantages of vanillin can be further extended as it functions as a building block for key intermediates for the synthesis of bio based polymers (Fache et al, 2016).
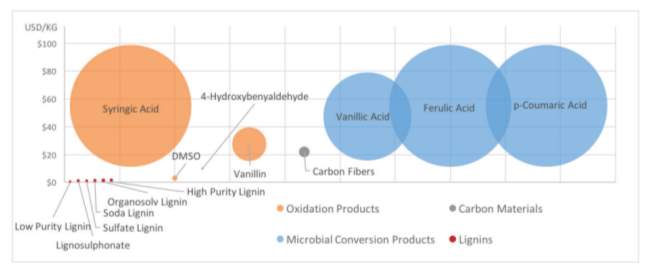
Figure. 5: Price of different forms of lignin, potential products and their derivatives. Individual bubbles represent minimum and maximum price of compounds. (Ľudmila et al, 2015).
This trend is explained by the fact that production of vanillin via natural routes is no longer feasible because existing growing areas are saturated and thus demand exceeds supply. Vanillin can also be produced chemically via the conversion of ferulic acid, which is not deemed as natural process. (Priefert et al, 2001). Consequently, releasing ferulic acid enzymatically from lignin is the current focus of research and highlights the importance of establishing the lignin degradation pathway in order to develop alternative, sustainable methods of production from lignin and possibly facilitate the production of novel chemicals. Comparing current applications of lignin, for example, as a precursor for carbon fibres with aromatics (Figure 5) highlights that current applications have a greater potential market value, explaining the vast research into optimising lignin degradation mechanism.
Moreover, upon investigating the effect of oxidation on lignin model compounds, vanillin yields over 40 % were achieved on account of their high content of β-O-4 linkages. On the contrary, upon oxidation of five typical lignin samples the highest vanillin yield obtained was 13.39 % (Wang et al, 2018). Despite the high vanillin yield obtained from lignin models, vanillin production directly remains challenging as the reproducible quantity that can be obtained is low. It is clear that there is more research required before this can expected to be achieved directly from raw biomass.
Lignin conversion technologies:
The following methods are currently used to degrade lignin in order to obtain value added chemicals. Liquefaction of lignin using hot-compressed water has been employed in order to yield phenolic feedstock under 3.4MPa and 260°C. (Yan et al, 2017). Moreover, hydrothermal degradation of lignin can also be used to produce phenolic compounds. Corncob lignin treated with pressurized hot water required a temperature of 290°C. This results in the formation of a heavy oil which subsequently serves as a starting material for phenol formaldehyde adhesive synthesis (Yang et al, 2015).
Pyrolysis involves the thermochemical degradation of lignin which is achieved by heating biomass to approximately 500°C in the absence of oxygen. Upon heating, subsequent cleavage of ether and carbon-carbon linkages occur to generate materials such as methanol, acetic acid, acetone (Laurichesse and Avérous, 2014). Additionally, an alternative method involves sulfonated residues obtained from lignin from sulphite pulping processes. This requires NaOH and Na2S and temperatures between 165°C-170°C (Laskar et al, 2013).
Lignin can also be degraded enzymatically by laccases due to their low redox potential. This facilitates their ability to directly oxidise lignin units. Laccases cannot penetrate biomass directly and therefore require a pre-treatment step such as the assistance from chemical oxidisers. (Martínez et al, 2005).
Taking into consideration the significant energy intensive requirements of the methods conventional methods outlined, the potential for superiority of photocatalysis as a method for degradation is clear. This is due to the fact that photocatalysis can operate optimally in ambient conditions
Mechanism of photocatalysis:
Photocatalytic processes combine photochemistry and catalysts to increase the rate of a chemical transformation (Serpone and Pelizzetti, 1989). A photocatalyst is defined as a ‘catalyst that accelerates the solar photo reaction’ that must adhere to the following specifications. The photocatalyst must not partake in or be consumed in the reaction and must also provide an alternative mechanism route with an increased reaction rate for existing photo reactions (Lee and Park, 2013). Photocatalysis has retained intense interest for many years because it utilises solar energy and can be implemented in mild conditions. It has remained relevant especially considering the current energy and environmental issues (Li et al, 2016). It is an Advanced Oxidation Process whereby the generation of a highly reactive transitory species such as OH∙ is used to mineralize a vast array of recalcitrant organic compounds (Munter, 2001).
Upon irradiation, a photon of equal or greater energy (hv) than the band gap of the semiconductor photocatalyst causes the promotion of a photoexcited electron from the filled valence band (VB) to the empty conduction band (CB) (Ohtani, 2014).

Figure 6. Electronic structure of semiconductor photocatalysts. (Ohtani,2013)
Following this, a photogenerated hole is (h+) is formed in the VB. This facilitates the following series of oxidative- reductive reactions that govern photocatlaysis using Titanium dioxide (TiO2) as the photocatalyst. Several semiconductors have been developed to exhibit photocatalytic behavior (Chong et al, 2010):
|
1. |
Photoexcitation |
TiO2 + hv → e– + h+ |
|
2. |
Charge-carrier trapping of e- |
e CB → e TR |
|
3. |
Charge carrier trapping of h+ |
h+ VB → h+ TR |
|
4. |
Electron-hole recombination |
e- TR + h+VB(h+TR) → e– CB + heat |
|
5. |
Photoexcited e scavenging |
(O2)ads + e → O∙2 |
|
6. |
Oxidation of hydroxyls: |
OH– + h+ → OH∙ |
|
7. |
Photodegradation by OH∙ |
R-H + OH∙ → R’∙ + H2O |
|
8. |
Direct photoholes |
R + h+ → R+ → Intermediate(s)/Final Degradation Products |
|
9. |
Protonation of superoxides |
O2∙− + OH∙ → HOO∙ |
|
10. |
Co-scavenging of e−: |
HOO∙ + e− → HO2− |
|
11. |
Formation of H2O2: |
HOO− + H+ → H2O2 |
Semi-conductor photocatalysis:
The formation of electron hole pairs is a result of the electronic structure of TiO2 which comprises of a filled valence band and an empty conduction band (Figure 6). The combination of electron holes which are characteristically strong oxidising agents and electrons which are strong reducing agents, promote redox reactions. Most reactions use the oxidising power of holes via OH∙ species (equation 6) and valence band holes to attack donor species such as organic molecules (equation 7) (Hernandez et al, 2009).

Figure 7. Photoinduced charge separation – mechanism of electron-hole pair formation in a TiO2 particle in the presence of pollutant in water (Umar and Aziz, 2013)
HOO∙ (equation 9) also have scavenging properties similar to oxygen, thus prolonging the photohole lifetime (equation 10 and 11).
Very fast recombination between electron and hole occurs unless oxygen (or any other electron acceptor) is available to scavenge the electrons to form superoxides (O2˙−) (Fig. 7), hydroperoxyl radicals (HO2˙) and subsequently H2O2.
Literature highlights an abundance of semi-conductors used in photocatalysis. The band positions of common catalysts are shown in Fig.4in relation to therequired potential of H2O splitting. TheEgof the catalysts are shown and as can beseen there are few catalysts which possess anEgsuitable for excitation by wave-lengths in the visible region of the electromagnetic spectrum Titanium Dioxide (TiO2) is most widely used on account prevailing advantages including low cost, toxicity, high chemical and thermal stability and availability (Zhao et al, 2016).
|
Semiconductor |
Valence band |
Conductance band (V vs NHE) |
Band gap wavelength (nm) |
|
TiO2 |
+3.1 |
-0.1 |
380 |
|
SnO2 |
+4.1 |
+0.3 |
318 |
|
ZnO |
+3.0 |
-0.2 |
390 |
|
ZnS |
+1.4 |
-2.3 |
336 |
|
WO3 |
+3.0 |
+0.2 |
443 |
|
CdS |
+2.1 |
-0.4 |
497 |
|
CdSe |
+1.6 |
-0.1 |
730 |
|
GaAs |
+1.0 |
-0.4 |
887 |
|
GaP |
+1.3 |
-1.0 |
540 |
Figure 8. Band positions of common semiconductor photocatalysts in aqueous solution at pH 1. (Robertson, 1996)

Figure 9. Band structure illustration of various semi-conductors with respect of the redox potentials of water splitting. (Jafari et al, 2016)
Upon physical mixing of cellulose and TiO2, no hydrogen was evolved, demonstrating that immobilization is a crucial aspect of the photocatalytic process. Moreover, investigation into the role of co-catalyst platinum showed lower CO2 and negligible hydrogen production in the absence of platinum. In summary, this highlights the approach that is required to obtain valuable sugars from cellulose (Zhang et al, 2016).
Conversely, hydrogen evolution from sugar, starch and cellulose in the presence of RuO2/TiO2/Pt photocatalyst showed an increase of 100 fold in comparison to the quantum yield obtained with TiO2 alone. This presumed to be due to strength of RuO2 as an electrode material (Kawai and Sakata, 1980). This method may be extended to decomposition of lignin with the simultaneous production of hydrogen.
An alternative strategy to increase photocatalytic performance uses Cadmium Sulfide quantum dots in an alkaline solution. This investigation showed that large volumes of H2 could be produced in the presence of strongly alkaline conditions namely 10M KOH, which simultaneously solubilizes a portion of cellulose. Increasing substrate available increased the rate of hydrogen evolution. Moreover, the activity of the CdS photocatalyst without the requirement of UV light and noble co-catalysts, had greater activity when compared with TiO2.
Heightened specific hydrogen evolution is due to higher solubility of hemicellulose, therefore more accessible oxidation sites for photocatalyst. Due to the fact that lignin is strongly light absorbing, the smaller band gap of CdS is advantageous in that this removes the competition for light, and allows for photocatalytic reaction to progress. This facilitated the production of quinones via oxidation of phenols within lignin (Wakerley et al, 2017).
Light source:
Although TiO2 is the most predominantly employed photocatalyst, it suffers from drawbacks such as having low efficiency of utilizing full spectrum of solar light. It is only capable of absorbing 5% of solar spectrum due to its wide band gap. To overcome this, alternative semi-conductors doping of particles have been investigated in order to effectively utilize visible light in photocatalytic reactions aforementioned.
Visible light mercury vapour lamps were explored as light sources for photocatalysis process. They emit at 2553.7nm and 184.9nm. Emission at 184.9nm has an absolute requirement for ultra-pure quartz. Moreover, medium pressure alternatives operate at 1-10 atmospheres require high temperatures compromising the intensity of the 257.9nm and 184.9nm spectra. (Horspool, 1976).
LED’s are preferred over conventional light sources as they offer intense light output allowing for faster degradation of organic contaminants. Increased output coupled with the ability to convert the majority of energy into light with little energy wasted as heat, makes LED’s a more cost-effective substitute. (Wang and Ku, 2005). LED’s are able to tune monochromatic light inferring the capacity to light source matched to absorption bands of sample of interest.
Reactor:
The design of photocatalytic reactors must fulfil two critical parameters, namely, they must provide uniform light distribution inside the reactor and providing high surface area for catalyst coating per unit of reactor volume. (Mukherjee and Ray, 1999). The issues associated with reactor design are due to upscaling for commercialisation application. The cost associated with constructing photoreactor systems capable of maintaining the selected optimum temperatures required to heat up solvents and high pressure systems that are required by some methods must be considered when selecting parameters for reactor designs. Moreover, the type of support used in photoreactor system determines the amount of immobilized catalyst and consequently the photoconversion rate, which must therefore be taken into account when desingin photoreactor systems. (Ola and MAroto-Valer, 2015). In summary, the ideal system be multi-functional; light transmission properties and also the ability to serve as catalyst carrier.
Cellulose:
Currently the success of photocatalysis for the degradation of organic waste is evident during the degradation of cellulose effluent in from paper mill. Cellulose is composed of D‐glucose monomers, linked by β‐1, 4 bonds. Cellulose is also difficult to dissolve without chemical modification because of the rigid long‐chain and strongly inter‐molecular and intra‐molecular hydrogen‐bonded structure. (Khazraji et al, 2013). One method used for photodegrading of cellulose involved a dissolving step in ZnCl2 solutionin a reactor coated with anatase TiO2. It was shown that ZnCl2 also assists with breaking down the glyosidic linkage facilitating further hydrolysis of the product monosaccharide. (Fan et al, 2011) Tehtrahydrofuran (THF) has been demonstrated to preferentially solvate lignin and therefore extract lignin from lignocellulose biomass. Despite both cellulose and lignin being major constituents in biomass and being characteristically recalcitrant, the lacking of a suitable co-solvent that readily solubilises lignin. However, THF is generally avoided as it does not adhere to green chemistry principles due to the fact that it is difficult to recycle, not environmentally friendly and also very hazardous substance due primarily to its flammable and explosive properties. (Xu et al, 2017).
Lignin model compounds
Considering that the lignin pathway is not well defined, often the method for prospecting the potential pathway looks at related compounds. The inert c-c bond of oxidized lignin model compounds was successfully converted to an active ester bond. This was achieved using Baeyer–Villiger (BV) oxidation, producing acetyl esters and aryl esters in high yields of up to 99% at room temperature. (Wang et al, 2017). This method requires THF, drawbacks as mentioned previously. By deriving knowledge of homologues, it is anticipated that this insight could be extended to assign possible mechanism for lignin. However, naturally derived lignin synthesized from several monomeric precursors of variable ratios means that findings obtained with lignin models cannot be directly applied to raw biomass. (Djikanović et al, 2012) Therefore, it is important to know how different combinations of lignin monomers impact structural changes, bond type and therefore degradation pathway. Obtaining a more comprehensive understanding about the degradation pathway of lignin may help to expand productivity and efficiency.
Current applications of Lignin in Pharmaceutical Industry:
Amongst the most novel applications of lignin in the pharmaceutical industry, is investigating the effect of tablets containing lignin on the release rate of acetylsalicylic acid. Increasing the release rate, effects tablet plasma concentrations and consequently improves bioavailability (Torrado et al, 1996). Results showed that acetylsalicylic acid tablets that incorporate lignin as an excipient, have an improved release rate compared to tablets without. Moreover, tablets containing lignin also displayed enhanced hardness and faster disintegration time (Pishnamazi et al, 2019). Optimising these parameters remains the focus of pharmaceutical research, thus, lignin proves attractive as a sustainable alternative. Controlling release rate infers many advantages, both, improving consumer compliance due to the reduced dosages required and providing a means of cost effective manufacturing (Nokhodchi et al, 2012). Furthermore, of £215 billion pharmaceutical market, that of the excipients is valued to amount to £1.5 billion. The most routinely used excipients include sugar, starch and cellulose derivatives (Pifferi and Restani, 2003).
Excipients are also employed as a method of compressing the formulation. Investigation of tablet compression behaviour of biomass components lignin and celluloses showed variation caused by difference in the source and isolation method used. (Penkina et al, 2013). Despite lignin displaying potential to function as an excipient, the World Health Organisation deems consistent purity and uniformity of chemical and physical characteristics as crucial for compliance with Good Manufacturing Practices. Thus, in order to warrant lignin as an economical substitute, further understanding of the mechanism of lignin degradation pathway is required considering the stringent regulations regarding excipients.
Incorporation of lignin and hemicellulose components into drug delivery systems blended with synthetic polymers have been shown to alter volume in response to surrounding stimuli with speed and efficiency. The cross-linked polymeric networks form hydrogels that have been proven useful as a means of absorbing biological fluids. Lignin is notoriously difficult to prevent from re-polymerisation (Farhat et al, 2017). It is evident that the substitution of synthetic polymers with lignin analogue has been explored. Conversely, the role of lignin degradation products and exploitation of these derivatives remains relatively new avenue of research.
References
- Belkhir, L., Elmeligi, A. (2018) ‘Carbon footprint of the global pharmaceutical industry and relative impact of its major players’ Journal of Cleaner Production, 214, pp. 185-194
- Boerjan, W., Ralph, J., Baucher, M. (2003) ‘Lignin Biosynthesis’ Annual Review of Plant Biology, 54, pp.519-546.
- Calvo-Flores, F.G., Dobado, J.A. (2010) ‘Lignin as Renewable Raw material’ Chemistry and sustainability Energy and Materials, 3(11) pp.1227-1235.
- Cherubini, F. (2010) ‘The biorefinery concept: Using biomass instead of oil for producing energy and chemicals’ Energy Conversion and Management, 51(7), pp. 1412-1421.
- Chong, M.N., Jin, B., Chow, C.W.K, Saint, C. (2010) ‘Recent developments in photocatalytic water treatment technology: A review, Water Research, 44(10), pp. 2997-3027
- Djikanović, D., Simonović, J., Savić, A., Ristić, I., Bajuk-Bogdanović, D., Kalauzi, A. (2012) ‘Structural Differences Between Lignin Model Polymers Synthesized from Various Monomers’ Journal of Polymers and the Environment, 20(2) pp. 607-617.
- Fache, M., Boutevin, B., Caillol, S. (2016) ‘Vanillin Production from Lignin and Its Use as a Renewable Chemical’ ACS Sustainable Chemistry and Engineering, 4(1), pp. 35-46.
- Farhat, W., Venditti, R., Mignard, N., Taha, M., Becquart, F., Ayoub, A. (2017) ‘Polysaccharides and lignin based hydrogels with potential pharmaceutical use as a drug delivery system produced by a reactive extrusion process’ International Journal of Biological Macromolecules, 104, (Pt A), pp. 564-575. doi: 10.1016/j.ijbiomac.2017.06.037
- Hatfield, R., Vermerris, W. (2001) ‘Lignin Formation in Plants: The Dilemma of Linkage Specificity’ Plant Physiology, 126, pp. 1351-1357.
- Hernández-Alonso, M.D., Fresno, F., Suárez, S., Coronado, J.M. (2009) ‘Development of alternative photocatalysts to TiO2: Challenges and opportunities’ Energy and Environmental Science, 2, pp. 1231-1257.
- Horspool, W.M. (1976) ‘Aspects of Organic Photochemistry’ New York: Academic Press.
- Jafari, T., Moharreri, E., Amin, A., S., Miao, R., Song, W., Suib, S.L. (2016) ‘Photocatalytic Water Splitting—The Untamed Dream: A Review of Recent Advances’ 21(7) pp.
- Jia, S., Cox, B.J., Guo, X., Zhang, Z.C., Ekerdt, J.G. (2010) ‘Cleaving the β–O–4 bonds of lignin model compounds in an acidic ionic liquid, 1-H-3-methylimidazolium chloride: an optional strategy for the degradation of lignin.’ Chemistry & Sustainability Energy & Materials, 3(9) pp. 1078-1084.
- Kaneko, Masao & Okura, Ichiro (2002) ‘Photocatalysis : science and technology ’, in 2002 Berlin: Kodansha. P. 900
- Kawai, T., Sakata, T. (1980) ‘Conversion of carbohydrate into hydrogen fuel by a photocatalytic process’ Nature, 286, pp. 474-476.
- Khazraji, A.C., Robert, S. (2013) ‘Self-assembly and Intermolecular Forces when Cellulose and Water Interact Using Molecular Modelling’ Journal of Nanomaterials, 2013, pp. 1-12.
- Laurichesse, S., Avérous, L. (2014) ‘Chemical modification of lignins: Towards biobased polymers’ Progress in Polymer Science, 39(7), pp. 1266-1290.
- Laskar, D.D., Yang, B., Wang, H. (2013) ‘Pathways for biomass‐derived lignin to hydrocarbon fuels’ Biofuels, Bioproducts & Biorefining, 7, pp. 602-626.
- Li, S.H., Liu, S., Colmenares, J.C., Xu, Y.J. (2016) ‘A sustainable approach for lignin valorization by heterogeneous photocatlaysis’ Green Chemistry, 18, pp. 594-607
- Lee, S.Y., Park, S.J. (2013) ‘TiO2 photocatalyst for water treatment applications’ Journal of Industrial and Engineering Chemistry, 19, pp.1761-1769
- L’udmila, H., Michal, J., Andrea, S., Ales, H. (2015) ‘Lignin, potential products and their market value’ Wood Research, 60(6) PP. 973-986.
- Martinez, A.T., Speranza, M., Ruiz-Duenas, F.G., Ferreora, P., Camarero, S., Guillen, F., Martinez, M.J., Gutierrez, A., del Rio, J.C. (2005) ‘Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin’ International Microbiology, 8, pp. 195-204.
- Mukherjee, P.S., Ray, A. K. ‘Major Challenges in the Design of a Large‐Scale Photocatalytic Reactor for Water Treatment’ Chemical Engineering and Technology, 22(3) pp. 253-260.
- Munter, R. (2001) ‘Advanced oxidation processes-current status and prospects’ Proceedings of the Estonian Academy of Sciences Chemistry, 50 (2001), pp. 59-80
- Nokhodchi, A., Raja, S., Patel, P., Asare-Addo, K. (2012) ‘The role of oral controlled release matrix tablets in drug delivery systems.’ Bioimpacts, 2(4), pp. 175–187. doi:10.5681/bi.2012.027
- Ohtani, B. (2013) ‘Titania Photocatalysis beyond Recombination: A Critical Review’ Catalysts, 3(4), pp. 942-953.
- Ohtani, B. (2014) ‘Revisiting the fundamental physical chemistry in heterogeneous photocatalysis: its thermodynamics and kinetics’ Physical Chemistry Chemical Physics, 16, pp.1788-1797.
- Ola, O., Maroto-Valer, M.M. (2015) ‘Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction’ Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 24, pp. 16-42.
- Pishnamazi, M., Iqbal,J., Shirazian, S., Walker, G.M., Collins, M.N. (2019) ‘Effect of lignin on the release rate of acetylsalicylic acid tablets’ International Journal of Biological Macromolecules, 124, pp. 354-359 doi.org/10.1016/j.ijbiomac.2018.11.136.
- Pifferi, G., Restani, P. (2003) ‘The safety of pharmaceutical excipients’ Il Farmaco, 58, (8), pp. 541-550.
- Priefert, H., Rabenhorst, J., Steinbuchel, A. (2001) ‘Biotechnoloigcal production of vanillin’ Applied Microbiology and Biotechnology, 56(3-4) pp.296-314.
- Rader, A. (2012) ‘Pharma and Healthcare Companies Find Local Emerging Market Conditions and Prices Challenging’ m brain. https://www.m-brain.com/white-papers/pharma-and-healthcare-companies-find-local-emerging-market-conditions-and-prices-challenging/
- Rahimi, A., Ulbrich, A., Coon, J.J., Stahl, S.S. (2014) ‘Formic-acid-induced depolymerisation of oxidized lignin to aromatics’ Nature, 515, pp. 249-252.
- Robertson, P.K.J. (1996) ‘Semi-conductor photocatalysis: an environmentally acceptable alternative production technique and effluent treatment process’ Journal of Cleaner Production, 4(3-4) pp. 203-212.
- Serpone, N., Pelizzetti, E. (1989) Photocatalysis: fundamentals and applications, New York: John Wiley & Sons.
- Sławomir, D. (2014) ‘Contemporary Trends in the Development of the Pharmaceutical Industry in the World’ Works of the Commission of Industrial Geography of the Polish Geographical Society, 25, pp. 108-131
- Speltini, A., Sturini, M., Dondi, D., Annovazzi, E., Maraschi, F., Caratto, V., Profumo, A., Buttafava, A. (2014) ‘Sunlight-promoted photocatalytic hydrogen gas evolution from water-suspended cellulose: a systematic study’ Photochemical & Photobiological Sciences, 13, pp. 1410-1419.
- Toledano, A., Serrano, L., Labidi, J. (2014) ‘Improving base catalyzed lignin depolymerization by avoiding lignin repolymerization’ Fuel, 116, PP.617-624
- Torrado, S., Cadorniga, R., Torrado, J.J. (1996) ‘Effect of drug release rate on bioavailability of different aspirin tablets’ International Journal of Pharmaceutics, 133 (1-2), pp. 65-70
- Umar, M. Aziz. H.A. (2013) ‘Photocatalytic Degradation of Organic Pollutants in Water. Organic Pollutants – Monitoring, Risj and Treatment
- Wahyudiono, Sasaki, M., Goto, M. (2008) ‘Recovery of phenolic compounds through the decomposition of lignin in near and supercritical water’ Chemical Engineering and Processing: Process Intensification, 47(9-10) pp. 1609-1619.
- Wang, W.Y., Ku, Y. (2005) ‘Photocatalytic degradation of Reactive Red 22 in aqueoussolution by UV-LED radiation’ Water Research, 40, pp. 2249-2258.
- Wang, Y., Wang, Q., He, J., Zhang, Y. (2017) ‘Highly effective C–C bond cleavage of lignin model compounds’ Green Chemistry’ 19(13), pp. 3135-3141.
- Wakerley, D.W., Kuehnel, M.F., Orchard, K.L., Ly, K.H., Rosser, T.E., Reisner, E. (2017) ‘Solar-driven reforming of lignocellulose to H2 with a CdS/CdOx photocatalyst’ Nature Energy, 2, pp. 1-10
- Xu, A., Guo, X., Zhang, Y., Li, Z., Wang, J. (2017) ‘Efficient and sustainable solvents for lignin dissolution: aqueous choline carboxylate solutions’ Green Chemistry, 19(17) pp. 4067-4073.
- Yan, L., Cui, Y., Gou, G., Wang, Q., Jiang, M., Zhang, S., Hui, D., Gou, J., Zhou, Z. (2017) ‘Liquefaction of lignin in hot-compressed water to phenolic feedstock for the synthesis of phenol-formaldehyde resins’ Composites Part B: Engineering, 112, PP.8-14.
- Yang, S., Yuan, T.Q., Li, M.F., Sun, R.C. (2015) ‘Hydrothermal degradation of lignin: Products analysis for phenol formaldehyde adhesive synthesis’ International Journal of Biological Macromolecules, 72, pp. 54-62.
- Zakzeski, J., Bruijnincx, P.C.A., Jongerius, A.L., Weckhuysen, B.M. (2010) ‘The Catalytic Valorization of Lignin for the Production of Renewable Chemicals’ Chemical Reviews, 110(6), pp. 3352-3599.
- Zhang, G., Ni, C., Huang, X., Welgamage, A., Lawton, L.A., Robertson, P.K.J., Irvine, J.T.S. (2016) ‘Simultaneous cellulose conversion and hydrogen production assisted by cellulose decomposition under UV-light photocatalysis’ Chemical Communications, 52(8), pp. 1673-1676.
- Zhao, H., Pan. F., Li, Y. (2016) ‘A review of the effects of TiO2 surface point defects on CO2 photoreductions with H20’ Journal of Materiomics, 3, pp. 17-32.
Website references:
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


