Mixed Solvent in CO2 Capture Technology
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2463 words | ✅ Published: 30 Nov 2017 |
Development of mixed solvent in CO2 capture technology with chemical absorption
- Xingye Fan
- Objectives:
This project mainly aims at developing mixed solvent in chemical absorption to achieve goals of increasing mass transfer rate, CO2 loading capacity, and reduce processing cost.
1.1 Short term objectives:
During the first five years, this project tends to test different mixed solvents to compare their property and performance in laboratory-scale. Mixed solvents with different components will be tested by a designed experiment in the laboratory. Advanced engineering process simulation will be performed by using Aspen plus software. By analyzing experiment and simulation data, the most suitable solvent for chemical absorption can be achieved.
1.2 Long term objectives:
If the solvent with promising property is obtained and the project continues beyond five years, performance of novel absorbent for CO2 capture can be tested in pilot-scale. If the application of the novel solvent in pilot-scale is proved to be feasible, this absorbent can be tried to be commercialized.
Chemical absorption processes are widely used to separate CO2 in coal fired power plants and chemical industries. Chemical absorption process is built on the reaction between the CO2 and chemical solvent. A typical chemical absorption process involves an absorber and a stripper. In this process, the flue gas which contains CO2 enters an absorber from the bottom and contacts with a CO2-lean absorbent counter-currently, after absorption, the CO2-rich absorbent flows to a thermally regenerator. In this method absorber and regenerator are working continuously. After regeneration, the CO2-lean stream is sent back to recycle for further use. The pure CO2 released from the regenerator is compressed and forwarded to storage or transportation. Due to the maturity of chemical absorption technology, it has been commercialized for a long time. Chemical absorption CO2 capture technologies are best utilized in post combustion because of its applicability for low CO2 concentration in the inlet gas stream. Chemical absorption is also considered as an efficient technique due to its low energy cost. By using individual type of solvent the absorption process has several drawbacks such as, the degradation of solvent, solvent regeneration efficiency, corrosion etc. which impact the efficiency of CO2 separation. To address the above problems, so much research has been conducted to improve solvent, modify gas-liquid contact device and prevent solvent degradation. Currently, to reduce the regeneration energy and further curtail the cost of absorption process, the operation of stripper is improved. Evidence shows that the operation of higher stripper pressure and alkanolamines concentration can be adopted to reduce energy consumption in regeneration. Volume of conventional absorption apparatus such as a packed bed, spray column, and a bubble column, is generally quite large. Therefore, small sizes of absorber and stripper with a lower equipment cost are expected. A rotating packed bed (RPB) was proposed which can also increase mass transfer rate between gas and absorbent.
CO2 capture technology with chemical absorption strongly depends on the performance of a liquid solvent. Thus, selecting a suitable solvent is the most effective method to improve the efficiency of this technique. So far, many researchers have focused on developing novel mixed solvents. Cullinane and Rochelle (2004) raised CO2 reaction rate by using potassium carbonate and amines with piperazine as a promoter. Rodrigo and Chakib (2010) improved the reaction rate by adding small amount of monoethanolamine or methyldiethanolamine into ammonia. Jeong Ho Choi and Seong Geun Oh (2012) increased CO2 reaction rate and CO2 loading capacity by mixed liquid solvent with 2-methylpiperidine as a promoter. Although so much research regarding to mixed solvent has been carried out, development of mixed solvents is still an essential research direction to improve absorption technology.
3 Method and proposed approach:
In order to measure the mass transfer rate of CO2 by using different solvents, we need to utilize a wetted wall column. Vapor-liquid equilibrium method is also used to evaluate the CO2 loading capacity. In addition, a simulation of the CO2 capture system can be developed by Aspen Plus software.
Work plan 1: measure mass transfer rate of CO2 with different mixed solvents (years 1-3)
In order to find a suitable combination of solvents, different kinds of absorbent mixture should be involved in the experiments. As the mass transfer rate of CO2 is a core parameter to determine property of absorbents, graduate students will carry out a wetted wall column experiment to measure CO2 mass transfer rate by using different mixed solvents. Solvents selection is of great significance. Various solvents are suitable for CO2 absorption such as monoethanolamine, diethylaniline, and methyldiethanolamine, K2CO3, Na2CO3, NaOH, NH3, Adenosine monophosphate. Alkanolamines are common absorbents for CO2 capture, and amines with different structure have various properties. Traditionally, alkanolamines can be classified into; primary, secondary, and tertiary amines. Among these three categories, the primary amines, for example monoethanolamine are considered the best solvent for flue gas cleaning because of the low partial pressure of CO2 in the flue gas. Monoethanolamine is a suitable solvent at low partial pressures of CO2 in the gas stream since it reacts quickly, and the cost of the raw materials is lower than secondary and tertiary amines. However, the operating cost of chemical absorption processes with monoethanolamine is high due to high energy cost in regenerating and operational problems such as corrosion, solvent loss, and solvent degradation. In addition, loading capacity of monoethanolamine can only be up to about 0.5mol of CO2/mol of monoethanolamine because of the formation of stable carbamates. Loading capacity of Tertiary alkanolamines such as methyldiethanolamine can reach 1mol of CO2/mol alkanolamine, and the energy consumption for regeneration is lower. However, the rates of CO2 absorption are low which make them not feasible for CO2 capture. A wide variety of alkanolamines that have proven to be commercially suitable for acid gas removal by chemical absorption are monoethanolamine, diethylaniline, methyldiethanolamine, and diglycolamine. The reaction of CO2 with primary and secondary alkanolamines to produce carbamates increases the CO2 interfacial mass transfer rate dramatically compared to the mass transfer rate without the chemical reactions and under the same driving force. However, because carbamate formation leads to the requirement of large amount of heat, the regeneration energy is significantly high. On the other hand, the slower reaction of tertiary amines with CO2 produces only bicarbonate and carbonate with a lower heat of reaction. Nevertheless, reaction with tertiary amines cannot raise the interfacial mass transfer rate to an ideal extent. Diglycolamine is also a primary amine that can be used at 50–70 wt% amines, leading to greatly lower circulation rates and energy requirements. The reactivity of diglycolamine is similar with monoethanolamine, but diglycolamine has a much lower vapor pressure. Thus, diglycolamine can be used in a more concentrated solution with less solvent flow rate. Therefore, according to the property of individual solvents, graduate students are required to select a diverse combination of solvents with different concentration and to measure their CO2 mass transfer rate with a wetted wall column. The construction of wetted wall column apparatus is described as the follows. The gas–liquid contactor in the center is constructed by a stainless-steel tube. The column is enclosed by a thick cylindrical wall glass and the whole chamber is surrounded by a second glass wall. Water flowing between the two glass walls can be used as a heat transfer medium. The absorbent is pumped into the column and flows down from the top and forms a thin liquid film along the outside surface of the column. Feed gas enters near the base of the chamber, counter-currently contacts with liquid and then exits from the top. During the experiment, the temperature in the chamber needs to be controlled to constant, and inside the reactor pressure is also maintained constant. The gas concentrations are measured with the non-dispersion infrared sensor continuously. Measurement of CO2 content in the inlet and outlet gas stream provides CO2 partial pressure and CO2 flux between gas and liquid. Other physical properties are analyzed by the different equipment such as density and viscosity is measured by density meter and viscometer respectively.
In the process of CO2 absorption, the molar flux of CO2 from the gas stream to the absorbents can be expressed as:
 (1)
(1)
In addition,
 =
= +
+ (2)
(2)
 is the gaseous molar flux of CO2. KG is the overall mass transfer coefficient, PCO2 and PCO2* are partial pressure of CO2 in the gas stream and at equilibrium in the liquid respectively. kG and kG’ are gas and liquid mass transfer coefficient respectively. kG‘ is a function of both the physical diffusion of the reactants in the liquid and the effect of the chemical reaction. In addition, the flux can be calculated if the contact area between the gas and the liquid as well as the amount of CO2 absorbed per unit of time is known. The flux can be calculated from equation (3):
is the gaseous molar flux of CO2. KG is the overall mass transfer coefficient, PCO2 and PCO2* are partial pressure of CO2 in the gas stream and at equilibrium in the liquid respectively. kG and kG’ are gas and liquid mass transfer coefficient respectively. kG‘ is a function of both the physical diffusion of the reactants in the liquid and the effect of the chemical reaction. In addition, the flux can be calculated if the contact area between the gas and the liquid as well as the amount of CO2 absorbed per unit of time is known. The flux can be calculated from equation (3):
 (3)
(3)
PCO2,in and PCO2,out are the partial pressure of CO2 in the inlet and outlet of the chambers which can be measured, P is the pressure in the chamber which can be measured by a pressure transducer, Qg is the flow rate of gas at the entrance of the chamber (m3/sec), including the water and solvent in the gas phase. The flow rates of water and solvent in the chamber are calculated with the thermodynamic model. Vm is the molar volume at the experimental conditions (mol/m3) and A is the contact area between the gas and the liquid. Therefore, by measuring the absorption flux at different partial pressures of CO2 and by using equation (1), it is possible to determine the overall mass transfer coefficient KG by plotting the flux as a function of the partial pressure of carbon dioxide in the chamber. After this part of experiment, we are able to screen some promising mixed solvents and carry out subsequent experiment with them.
Work plan 2: evaluate CO2 loading capacity of different mixed solvents (years 3-4)
In this section, graduate students need to measure CO2 loading capacity of the left mixed solvent with vapor-liquid equilibrium system. The vapor–liquid equilibrium system includes a CO2 supplier, a reactor, a measuring device that indicates temperature and pressure, and a computer that records CO2 pressure immediately. The reactor is batch reactor with a magnetic stirrer at the bottom to increase the gas–liquid contact area.
Equilibrium partial pressure of CO2 in the reactor can be expressed as follows:
P*CO2=P*-P0 (4) P* is the equilibrium pressure at the absorption equilibrium and P0 is the initial pressure. The mole of the CO2 entered can be calculated by the ideal gas law as follows:
nSCO2= (5)
(5)
PSi is the initial pressure of supplier. PSt is the pressure of supplier after injection of CO2. VS is the volume of supplier, TS is the temperature of supplier and R is gas constant. The mole of gaseous CO2 in the reactor at equilibrium can also be determined by the ideal gas law.
nRCO2= (6)
(6)
In the above equation, PRi is the initial pressure of reactor. PRt is the pressure of reactor after equilibrium VR, TR are volume of reactor and temperature of reactor, respectively. Eventually, the total amount of absorbed CO2 can be determined by Eq (7).
nabsorbedCO2=nSCO2-nRCO2 (7)
The loading capacity can be expressed by molar solubility which is the mole of the CO2 absorbed divided by the mole of absorbent:
NCO2loading=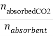 (8)
(8)
By comparing CO2 loading capacity, we are able to get rid of some mixed solvents with poor CO2 loading capacity. Then, left mixed solvents are selected for further research. The concentration of mixed solvents is also very important. In order to get the specific concentration at which mixed solvent can work best, wetted wall column experiments and vapor-liquid equilibrium experiments are required to conduct repetitively.
Work plan 3: Simulation of process (years 4-5)
In case, we can obtain suitable mixed solvents from above sections and according to the kinetics study in work plan 1, graduate students will be assigned to simulate the process of CO2 capture pilot plant using chemical absorption method. The simulation is manipulated with Aspen plus Software. The objectives of this work are as follows: Firstly, by carrying out the simulation, we can collect the data of CO2 removal efficiency. Besides, the simulation can help to determine the energy consumption in the CO2 capture pilot plant. Based on these data, we can screen the mixed solvents which can reduce the processing cost. Moreover, the simulation of the process is also an efficient way to evaluate a capture process and to optimize the process in order to reduce the heat, water and electricity consumption. At last, when further research is done such as test the solvent performance in a pilot plant, we can compare the data collected from the pilot plants with simulation data to perform the verification.
4 Anticipated significance of the work
After devoting over five years to this project, we hope to find a better absorbent by developing mixed solvent in CO2 capture technology. This outcome will not only increase the efficiency of the chemical absorption CO2 capture technology but also reduce the energy consumption of this technology. Since the chemical absorption technology is widely used for CO2 capture, the discovery of an innovative solvent will definitely make this technology more competitive.
5 Training for graduate students and researchers
This project will develop graduate students’ skills of carrying out wetted water column and vapor-liquid equilibrium experiments as well as the ability to calculate mass transfer rate and CO2 loading capacity. In addition, graduate students will also obtain the skills of processing and analyzing data. This project also requires students have skills of using software related to chemical engineering such as Aspen plus. The working experience on the project will provide students ability of performing multi-task, creativity, critical thinking ability, detail-oriented characteristic. The ability will be significantly helpful for their future career and will make them competitive among peers.
Reference
[1] Jinzhao Liu. Study on mass transfer and kinetics of CO2 absorption into aqueous ammonia and piperazine blended solutions [J].Chemical Engineering Science, 2012, 75: 298-308.
[2] Hendy Thee, Yohanes A. Suryaputradinata, Kathryn A. A kinetic and process modeling study of CO2 capture with MEA-promoted potassium carbonate solutions [J]. Chemical Engineering Science, 2012, 210: 271-279.
[3] Victor Darde. CO2 capture using aqueous ammonia: kinetic study and process simulation [J]. ScienceDirect, 2011,4: 1443-1450.
[4] Dey A, Aroonwilas A. CO2 absorption into MEA-AMP blend: mass transfer and absorber height index. Energy Procedia 2009.
[5] Mondal MK. Solubility of carbon dioxide in an aqueous blend of diethanolamine and piperazine. Journal of Chemical & Engineering Data 2009;54: 2381e5.
[6] Lepaumier H, Martin S, Picq D, Delfort B, Carrette PL. New amines for CO2 capture III, effect of alkyl chain length between amine functions on polyamines degradation. Industrial & Engineering Chemistry Research 2010; 49:4553e60.
[7] Electric Power Research Institute (EPRI). Post-combustion CO2 capture technology
[8] Yaser Khojasteh Salkuyeh. Reduction of CO2 capture plant energy requirement
by selecting a suitable solvent and analyzing the operating parameters [J]. Energy Research, 2012, 37: 973-981.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


