Metal Complex Formation in Wastewater Systems
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1484 words | ✅ Published: 18 May 2020 |
‘Metal Complex Formation in Wastewater Systems’
Introduction
Wastewater comprises domestic, industrial or commercial used water. It is a combination of the greywater (water from baths, sinks, washing machines and kitchen sources) and blackwater (a mixture of urine, feces and flush water). Chemistry of wastewater is very interesting as it includes ions, organic chemicals, heavy metals, metalloids, and microplastics. We are particularly interested in studying metal complexes in wastewater. Complex ion has metal at its center with some ions or molecules attached to a central atom with coordinate bonds. [1]An ion or molecule attached to a metal atom is known as Ligand.
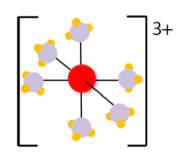

Fig: A Chromium (Cr) metal is surrounded by ammonia molecules forming complex ion. [2]
This case study would be considering heavy metal and it’s complex structures. Heavy metals refer to chemical structures that have relatively high density and is toxic or poisonous at lower concentration. Examples : Mercury(Hg), Cadmium(Cd), Arsenic(As), Lead(Pb), Chromium(Cr) etc. They have the property to bioaccumulate and it’s concentration rises over time.
Important Disasters with Metals
a) Minamata disaster
This disaster occurred in 1932 in Minamata City, south-west region of Japan’s Kyushu Island. However, it was officially discovered that marine products in Minamata Bay displayed high levels of Hg contamination (5.61 to 35.7 ppm). Chisso Co. Ltd discharged containing MeHg contaminated wastewater in the surrounding leading to Minamata disease affecting a larger population in that area. [3]
b) Donana Disaster
This disaster occurred in 1998 in Andalusia, southern Spain when a holding dam burst at the mine releasing dangerous levels of several heavy metals and its complexes. (First citation in the Wikipedia). Since, the waste was highly acidic, which contained a mixture of lead, copper, zinc, cadmium and other metals. It had a greater ecological impact, and nothing survived. (Wikipedia 6th citation).
Metal Complexes in Natural Water Systems
Natural water is classified as a water system that has less than 20 mg l-1 of organic matter or water that has total organic concentrations as high as 100 mg l-1. [4] The rationale behind studying metal complexes in the natural water system to obtain data amendable to biology like toxicity to aquatic organisms. [5] Later, the concept of metal complexes in natural systems help to extract metals using the precipitation by developing the concept of stability concepts for metal-ligand complexes. By changing the chemistry of complexation in an organism, it would be helpful to retain a pool of free metal ion, which aids in the new field of bioremediation. (Book: Water Chemistry: Second edition, page number 549-556).
Metal Complexes in Wastewater Systems
Relatively, little attention to heavy metal speciation in samples such as sewage, sewage effluent, and sludge was given until the 1980s due to matrix interference effects and uncertainty about the validity of sensitive analytic techniques applied to these metal complexes. [6] The study of metal complexes in wastewater started getting into the radar around the 1980s as it helps researchers and scientists understand the partitioning of heavy metals into various forms, which eventually help in determining dispersion and impact in the environment. The importance of metal speciation has been recognized in anaerobic wastewater treatment as it appears that biological processes are susceptible to metal toxicity [7] For sewage sludge, the importance of heavy metals and its speciation influences physiochemical forms of heavy metals and their bioavailability [8] It has been considered to use as an application to explore and manipulate cell biology [9] Characterizing ligands from metals originating from different sources in wastewater effluent has been a challenge and there are researchers, who believe that solving this problem can open a new field in wastewater-based epidemiology (WBE). [10]
Analytical Chemistry, Separation Methods, and Limitations
There are two different approaches involved in understanding the speciation of metal complexes in wastewater. The first one is analytical chemistry, which is used to study one or multiple groups of species present in many other metal forms. The second alternative method is the separation method, which is used to isolate species of interest before determining the metal concentration. [6]
Many analytical methods have been used throughout the scientific arena, but few popular ones will be discussed here along with the potential. Direct determination by ion-specific electrodes (ISEs) is one of the oldest yet popular method that has been used to determine free metal in systems where a variety of metal species exist. Since the calibration is simple and it almost gives a direct readout of free metal ions, it is very easy to use.[6] However, there have been questions on what kind of electrodes to use while experimenting with electrodes may respond to other metals present more than limiting concentrations.[11] Interferences have been detected by [12] while working with copper metal speciation. [Cu2+] selective electrode gives a positive response in solutions of citrate, tartrate in the absence of copper [13]
Advanced analytical techniques like Size Exclusion Chromatography (SEC) coupled to an ICP-MS also has its own limitations as it detects for multiple elements simultaneously like complexes of P-species in the environment and mobile phase can interact with the column giving uncertainties [14] Studies using ion-exchange methods demonstrated that principal organic ligands in sewage most likely multidentate and neutral charges like NH3 have lower affinity for species with lesser like Ni2+. Developing specific ion-exchange techniques was brought inconsistency in the metal concentrations [15].
Importance of Metal Complexation
It helps in understanding the fate and transport of the heavy metals and the species persisting in our ecosystem. It also helps to understand the interaction of metals with different kinds of ligands, facilitate the hydrolysis reactions, magnetic properties of transition metals and bioremediation processes [9]. 10 different metals from their complex structures would help in the interpretation of the relationship between metal exposure and population health through the use of wastewater-based epidemiology (WBE) [10] .
Environmental Engineering Chemistry and Metal Complexation in Wastewater Systems
Chemistry of the metal complexation emphasizes the importance of method validation while performing any experiments. It is evidence of how science has evolved and the scientific advancement that our generation has achieved. It highlights the difference between the natural system and the engineered system, and how chemistry could change with different matrices. Metal complexes study helps in extracting metals from the wastewater and paddles towards sustainability promoting a circular economy. A model to capture the relative potential for economic value of 13 metals with a combined value of sludge worth U.S. $280 per ton of sludge. [16]
[1] “Metal Complexes” [Online]. Available: https://chem.libretexts.org/Courses/Saint_Mary’s_College%2C_Notre_Dame%2C_IN/CHEM_342%3A_Bio-inorganic_Chemistry/Readings/Week_2%3A_Introduction_to_Metal-Ligand_Interactions/2.1_Introduction_to_metal_complexes/
[2] B. M, Water chemistry. Grooveland: Waveland Press Inc.
[3] M. Harada, “Critical Reviews in Toxicology Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution,” Crit. Rev. Toxicol., 1995.
[4] J. H. Reuter and E. M. Perdue, “Importance of heavy metal-organic matter interactions in natural waters,” Geochim. Cosmochim. Acta, 1977.
[5] T. A. Neubecker and H. E. Allen, “The measurement of complexation capacity and conditional stability constants for ligands in natural waters,” Water Research. 1983.
[6] “complexing sites available would be dominated by major cations , such as Ca 2 ÷ , Mg 2 ÷ , Fe 2 + and Fe B ÷ , and there is little scope for the inclusion of ill-defined organic moieties in speciation models . Natural waters may generally be classified as,” vol. 34, pp. 117–141, 1984.
[7] F. E. Mosey, “Assessment of the maximum concentration of heavy metals in crude sewage which will not inhibit the anaerobic digestion of sludge,” Water Pollut. Control, 1976.
[8] J. N. Lester, R. M. Sterritt, and P. W. W. Kirk, “Significance and behaviour of heavy metals in waste water treatment processes II. Sludge treatment and disposal,” Sci. Total Environ., 1983.
[9] K. L. Haas and K. J. Franz, “Application of metal coordination chemistry to explore and manipulate cell biology,” Chem. Rev., vol. 109, no. 10, pp. 4921–4960, 2009.
[10] C. Markosian and N. Mirzoyan, “Wastewater-based epidemiology as a novel assessment approach for population-level metal exposure,” Sci. Total Environ., vol. 689, pp. 1125–1132, 2019.
[11] G. J. Moody and J. D. R. Thomas, “Selective ion-sensitive electrodes,” Sel. Annu. Rev. Anal. Sci., 1973.
[12] J. Barica, “Unusual Response of a Cupric Ion Electrode in Prairie Lake Waters,” J. Fish. Res. Board Canada, 1978.
[13] M. F. El-Taras, E. Pungor, and G. Nagy, “The influence of some organic complexing agents on the potential of copper(II) – selective electrodes. application of the silicone rubber-based electrode to the determination of citrate ion and 8-hydroxyquinoline,” Anal. Chim. Acta, 1976.
[14] A. K. Venkatesan, W. Gan, H. Ashani, P. Herckes, and P. Westerhoff, “Size exclusion chromatography with online ICP-MS enables molecular weight fractionation of dissolved phosphorus species in water samples,” Water Res., vol. 133, pp. 264–271, 2018.
[15] F. F. Cantwell, J. S. Nielsen, and S. E. Hrudey, “Free Nickel Ion Concentration in Sewage by an Ion Exchange Column-Equilibration Method,” Anal. Chem., 1982.
[16] P. Westerhoff et al., “Characterization, Recovery Opportunities, and Valuation of Metals in Municipal Sludges from U.S. Wastewater Treatment Plants Nationwide,” Environ. Sci. Technol., vol. 49, no. 16, pp. 9479–9488, 2015.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


