Is Premium Grade Fuel More Efficient?
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2056 words | ✅ Published: 23 Sep 2019 |
What is Gasoline?
Gasoline is made up of molecules made up of hydrogen and carbon atoms arranged in chains. Gasoline molecules have anywhere from, 7 to 11 carbon atoms in each chain. When you burn gasoline under STP, you get carbon dioxide. Which is from the carbon atoms within the gasoline. A litre of Advance grade Gasoline contains about 1.3 x 108 joules of energy, which is equivalent to 123,000 BTU or 36,047 watt-hours. If it were possible for human beings to digest gasoline, a litre would contain about 31,070 food calories, the energy in a litre of gasoline is equivalent to the energy in about 130 McDonalds hamburgers.
Where does gasoline come from?
Gasoline is made from crude oil which is drilled from the earth. This crude oil consists of organic material that has been decomposing over millions of thousands of years. The crude oil is then pumped out of the ground and is then refined to get different resources, Petroleum. Petroleum is a liquid that contains hydrocarbons which is used to make gasoline. All hydrocarbon molecules of different lengths have different properties. ex, a chain with just one carbon atom is, (CH4-Methane) it is the lightest chain. As the chains get longer, they get heavier. CH4 (methane), C2H6 (ethane), C3H8 (propane) and C4H10 (butane) are all gases, and they boil at -161, -88, -46 and -1 degrees F, (-107, -67, -43 and -18 degrees C). The chains C5H12 up through C18H32 are all liquids at STP, and the chains above C19 are all solids at STP. The different chain lengths have progressively higher boiling points, so they can be separated out by distillation. This is what happens in an oil refinery, crude oil is heated and the different chains are pulled out by their vaporization temperatures. The chains in the standard range are all very light and are easily vaporized and create clear liquids called naphthas. Dry, cleaning fluids can be made from these liquids, as well as paint solvents and other quick-drying products. The chains from C7H16 through C11H24 are blended together and used for gasoline. All of them vaporize at temperatures below the boiling point of water. That’s why if you spill gasoline on the ground it evaporates very quickly. Next is kerosene, in the C12 to C15 range, followed by diesel fuel C16 – C18 and heavier fuel oils (like heating oil for houses). Next come the lubricating oils. These oils no longer vaporize in any way at standard temperatures. For example, engine oil can run all day at 250 degrees F (121 degrees C) without vaporizing at all. Oils go from various viscosity from motor oil which is able to pass through the very high gear oils and then semi-solid greases. Vaseline falls in there as well. Chains above the C20 range form solids, starting with paraffin wax, then tar and finally asphaltic bitumen, which used to make asphalt roads. All of these different substances come from crude oil. The main difference is the length of the carbon chains.
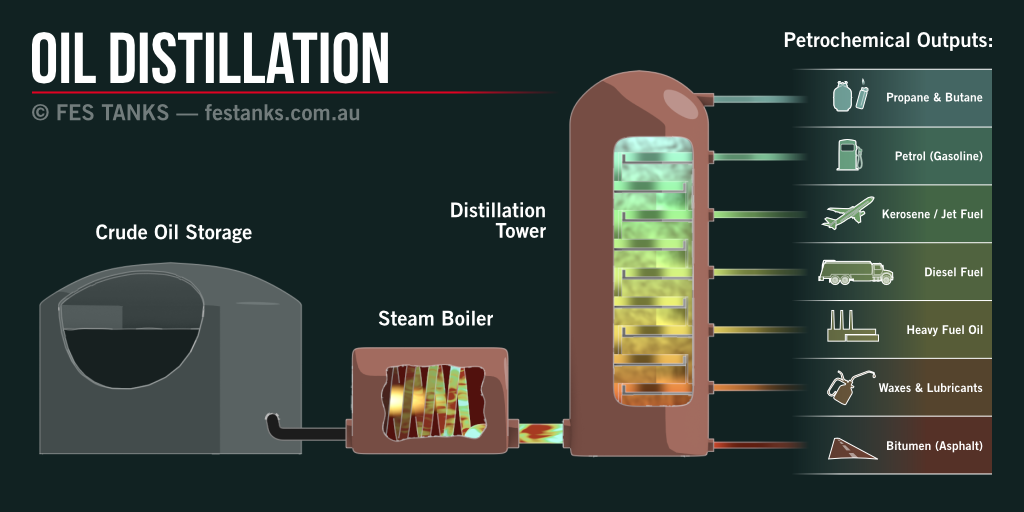
What is Octane?
Almost all cars use four-stroke gasoline engines. One of the strokes is the compression stroke, where the engine compresses a cylinder-full of air and gas into a much smaller volume before igniting it with a spark plug. The amount of compression is called the compression ratio of the engine. A typical engine might have a compression ratio of 8-to-1. The octane rating of gasoline tells you how much the fuel can be compressed before it spontaneously ignites. When gas ignites by compression rather than because of the spark from the spark plug, it causes knocking in the engine. Knocking can damage an engine, so it is not something you want to have happening. Lower-octane gas (like “regular” 87-octane gasoline) can handle the least amount of compression before igniting. The compression ratio of your engine determines the octane rating of the gas you must use in the car. One way to increase the horsepower of an engine is to increase its compression ratio and the displacement of the engine. So a “high-performance engine” has a higher compression ratio and requires higher-octane fuel. The advantage of a high compression ratio is that it gives your engine a higher horsepower rating for a given engine weight, that is what makes the engine “high performance”, which means the engine is more efficient. The disadvantage is that the higher octane gasoline for your engine costs more. The name “octane” comes from when you take crude oil and “crack” it in a refinery, you end up getting hydrocarbon chains of different lengths. These different chain lengths can then be separated from each other and blended to form different fuels. For example, methane, propane and butane are all hydrocarbons. Methane has a single carbon atom. Propane has three carbon atoms chained together, etc. It turns out that heptane handles compression very poorly. Compress it just a little and it ignites spontaneously. Octane handles compression very well, you can compress it a lot and nothing happens. 87 octane gasoline is gasoline that contains 87-percent octane and 13-percent heptane (or some other combination of fuels that has the same performance of the 87/13 combination of octane/heptane). It spontaneously ignites at a given compression level, and can only be used in engines that do not exceed that compression ratio.
Table 1: Grades of fuel
|
Grade |
Octane |
% Ethanol |
Carbon chain range |
Density g/ml |
|
Standard |
87 |
10 |
C7H16 – C11H24 |
0.73 |
|
Advanced |
89 |
5 |
C7H16 – C11H24 |
0.73 |
|
Premium |
91 |
0 |
C7H16 – C11H24 |
0.73 |
|
Diesel |
12 |
0 |
C16H34 – C18H38 |
0.84 |
Question: Is there value in buying higher grade fuel? Comparison of fuel grades using ∆H?
Hypothesis: Premium gas which has a higher octane rating will be more efficient than standard gas. Premium gas contains less ethanol, which has short chains, so has more longer chains which release more energy.
Independent variable: Grades of fuel, Standard, Advanced, Premium ,and Diesel
Dependent variable: Temperature change
Other measured: Mass of fuel burned
Sample collections and plus transport:
Materials:
- 1 jerry can
Collection of fuel from gas station:
Method:
– Clean and dry jerry can, starting from
– Collect samples of fuel from gas stations
– Transport to lab
– Approximately measure 150ml of each fuel into a clean beaker.
Experiment
Materials:
● Thermometer
● Thermometer clamp
● Stand
● Spirit burner with wicks
● Glass erlenmeyer flask
● 200ml Water
● Clamp
● Funnel
● Lighter
● Timer
● Graduated cylinder
Method:
- Pour approximately 150 ml of gasoline in a clean dry 250ml beaker.
- Place ‘50 ml’ of fuel into clean, dry spirit burner in fume hood.
- Weigh the filled fuel burner.
- Measure and 200 cm3 of water into beaker.
- Clamp beaker 15 cm above level ground.
- Place thermometer in thermometer clamp.
- Place thermometer in beaker and record initial temperature.
- Place spirit burner under beaker plus light wick.
- After 5 minutes record final temperature and extinguish flame.
- Reweigh spirit burner.
- Record data in table.
- Repeat step 2-11, 9 more times with fuel sample.
- Clean and dry spirit burner, let wick dry out.
- Repeat steps 1-13 for other fuel samples.
Experimental results
Table 2: Experimental results and differences of mass and temperature
|
Grade of Fuel and trial number |
Initial fuel mass(g)± 0.01 |
Final fuel mass(g)± 0.01 |
Initial temp(°C) ±0.5 |
Final temp(°C) ±0.5 |
Difference of mass (g)±0.02 |
Difference of temp (°C)±1 |
|
Standard #1 |
187.21 |
181.27 |
21 |
97 |
5.94 |
76 |
|
Standard #2 |
187.18 |
183.51 |
22 |
98 |
3.67 |
76 |
|
Standard #3 |
187.42 |
182.26 |
22 |
99 |
5.16 |
77 |
|
Standard #4 |
187.23 |
180.94 |
19 |
99 |
6.29 |
80 |
|
Standard #5 |
186.68 |
180.02 |
20 |
98 |
6.66 |
78 |
|
Standard #6 |
186.76 |
180.76 |
21 |
93 |
6 |
72 |
|
Standard #7 |
186.24 |
180.59 |
20 |
92 |
5.65 |
72 |
|
Standard #8 |
186.11 |
180.14 |
20 |
97 |
5.97 |
77 |
|
Standard #9 |
186.57 |
179.88 |
21 |
97 |
6.69 |
76 |
|
Standard #10 |
186.58 |
180.62 |
16 |
90 |
5.96 |
74 |
|
Advanced #1 |
184.62 |
179.7 |
25 |
88 |
4.92 |
63 |
|
Advanced #2 |
185.49 |
180.38 |
18 |
85 |
5.11 |
67 |
|
Advanced #3 |
184.58 |
178.6 |
18 |
87 |
5.98 |
69 |
|
Advanced #4 |
184.94 |
179.52 |
19 |
86 |
5.42 |
67 |
|
Advanced #5 |
185.29 |
180.23 |
17 |
88 |
5.06 |
71 |
|
Advanced #6 |
184.97 |
179.97 |
20 |
88 |
5 |
68 |
|
Advanced #7 |
185.37 |
180.9 |
20 |
88 |
4.47 |
68 |
|
Advanced #8 |
185.1 |
180.27 |
18 |
87 |
4.83 |
69 |
|
Advanced #9 |
185.01 |
180.1 |
18 |
88 |
4.91 |
70 |
|
Advanced #10 |
184.07 |
179.24 |
19 |
89 |
4.83 |
70 |
|
Premium #1 |
184.98 |
178.92 |
18 |
66 |
6.06 |
48 |
|
Premium #2 |
185.57 |
178.34 |
19 |
70 |
7.23 |
51 |
|
Premium #3 |
185.26 |
178.62 |
17 |
65 |
6.64 |
48 |
|
Premium #4 |
185.57 |
179.58 |
20 |
67 |
5.99 |
47 |
|
Premium #5 |
185.35 |
179.86 |
18 |
74 |
5.49 |
56 |
|
Premium #6 |
184.82 |
179.27 |
19 |
72 |
5.55 |
53 |
|
Premium #7 |
184.87 |
179.57 |
12 |
57 |
5.3 |
45 |
|
Premium #8 |
184.07 |
180.05 |
15 |
67 |
4.02 |
52 |
|
Premium #9 |
184.38 |
179.99 |
15 |
69 |
4.39 |
54 |
|
Premium #10 |
185.02 |
180.32 |
16 |
66 |
4.7 |
50 |
|
Diesel #1 |
192.85 |
187.67 |
21 |
54 |
5.18 |
33 |
|
Diesel #2 |
193.27 |
185.89 |
20 |
53 |
7.38 |
33 |
|
Diesel #3 |
192.92 |
186.25 |
22 |
57 |
6.67 |
35 |
|
Diesel #4 |
192.76 |
185.76 |
19 |
52 |
7 |
33 |
|
Diesel #5 |
193.17 |
185.99 |
18 |
49 |
7.18 |
31 |
|
Diesel #6 |
193.02 |
187.82 |
16 |
46 |
5.2 |
30 |
|
Diesel #7 |
193.32 |
187.52 |
19 |
51 |
5.8 |
32 |
|
Diesel #8 |
192.76 |
185.81 |
20 |
52 |
6.95 |
32 |
|
Diesel #9 |
192.98 |
186.99 |
22 |
60 |
5.99 |
38 |
|
Diesel #10 |
192.87 |
186.02 |
21 |
57 |
6.85 |
36 |
Analysis
To calculate q, we use q = M*c(constant)*∆T. Calculating heat change, q= the mass of water, 200 g, multiplied by the specific heat capacity of water multiplied by the change in temp. Then we calculate the heat change per 1 L .Finally we Average and % error for ∆H.
Calculating heat change in KJ
q = m c ∆T
= (200g)(4.18 J g-1 °C-1)(76°C)
= 90288 J / 1000
= 90.288
Calculating heat change per litre
D = m/v => v = m/D
Standard #1 Density For gasoline Diesel #1 Density for diesel
For gasoline: v = 5.94 g / 0.73 g/mol For Diesel: 5.18 g / 0.84g/mol
Average Calculation
Add all V from above for each fuel and divide by 10, since there are 10 samples
(11096 + 11096 + 11242 + 11680+ 11388 + 10512 + 10512 + 11242 + 11096 + 10804) / 10 =11066.8 which is average heat change per liter.
Minimum calculation
Subtract average heat change by heat change of trial to get a positive or negative
11066.8 – 10512 = 554.8
Find largest negative number to get minimum calculation
|
Standard #6 |
6 |
72 |
86 |
8.2 |
10512 |
-554.8 |
Table 3: Calculation of heat change and heat change per Litre:
|
Grade of Fuel and trial number |
Difference of mass (g)±0.02 |
Difference of temp (°C)±1 |
Heat Change(q) KJ |
Change in Volume mL |
heat change per L KJ/L |
|
Standard #1 |
5.94 |
76 |
90 |
8.1 |
11096 |
|
Standard #2 |
3.67 |
76 |
56 |
5.0 |
11096 |
|
Standard #3 |
5.16 |
77 |
79 |
7.1 |
11242 |
|
Standard #4 |
6.29 |
80 |
101 |
8.6 |
11680 |
|
Standard #5 |
6.66 |
78 |
104 |
9.1 |
11388 |
|
Standard #6 |
6 |
72 |
86 |
8.2 |
10512 |
|
Standard #7 |
5.65 |
72 |
81 |
7.7 |
10512 |
|
Standard #8 |
5.97 |
77 |
92 |
8.2 |
11242 |
|
Standard #9 |
6.69 |
76 |
102 |
9.2 |
11096 |
|
Standard #10 |
5.96 |
74 |
88 |
8.2 |
10804 |
|
Advanced #1 |
4.92 |
63 |
62 |
6.7 |
9198 |
|
Advanced #2 |
5.11 |
67 |
68 |
7.0 |
9782 |
|
Advanced #3 |
5.98 |
69 |
83 |
8.2 |
10074 |
|
Advanced #4 |
5.42 |
67 |
73 |
7.4 |
9782 |
|
Advanced #5 |
5.06 |
71 |
72 |
6.9 |
10366 |
|
Advanced #6 |
5 |
68 |
68 |
6.8 |
9928 |
|
Advanced #7 |
4.47 |
68 |
61 |
6.1 |
9928 |
|
Advanced #8 |
4.83 |
69 |
67 |
6.6 |
10074 |
|
Advanced #9 |
4.91 |
70 |
69 |
6.7 |
10220 |
|
Advanced #10 |
4.83 |
70 |
68 |
6.6 |
10220 |
|
Premium #1 |
6.06 |
48 |
58 |
8.3 |
7008 |
|
Premium #2 |
7.23 |
51 |
74 |
9.9 |
7446 |
|
Premium #3 |
6.64 |
48 |
64 |
9.1 |
7008 |
|
Premium #4 |
5.99 |
47 |
56 |
8.2 |
6862 |
|
Premium #5 |
5.49 |
56 |
61 |
7.5 |
8176 |
|
Premium #6 |
5.55 |
53 |
59 |
7.6 |
7738 |
|
Premium #7 |
5.3 |
45 |
48 |
7.3 |
6570 |
|
Premium #8 |
4.02 |
52 |
42 |
5.5 |
7592 |
|
Premium #9 |
4.39 |
54 |
47 |
6.0 |
7884 |
|
Premium #10 |
4.7 |
50 |
47 |
6.4 |
7300 |
|
Diesel #1 |
5.18 |
33 |
34 |
6.2 |
5544 |
|
Diesel #2 |
7.38 |
33 |
49 |
8.8 |
5544 |
|
Diesel #3 |
6.67 |
35 |
47 |
7.9 |
5880 |
|
Diesel #4 |
7 |
33 |
46 |
8.3 |
5544 |
|
Diesel #5 |
7.18 |
31 |
45 |
8.5 |
5208 |
|
Diesel #6 |
5.2 |
30 |
31 |
6.2 |
5040 |
|
Diesel #7 |
5.8 |
32 |
37 |
6.9 |
5376 |
|
Diesel #8 |
6.95 |
32 |
44 |
8.3 |
5376 |
|
Diesel #9 |
5.99 |
38 |
46 |
7.1 |
6384 |
|
Diesel #10 |
6.85 |
36 |
49 |
8.2 |
6048 |
Graph 1: Average heat change per litre of 4 different grades of fuel:
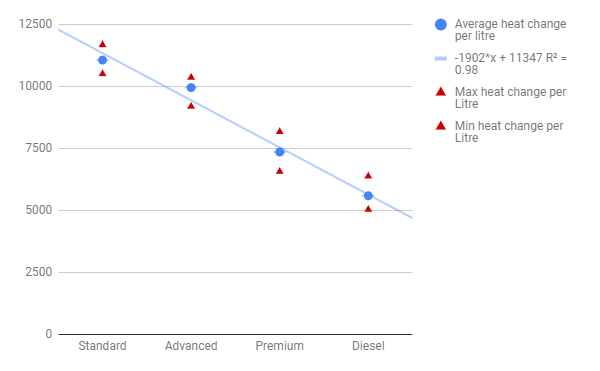
Table 4: Average change of heat per Litre and maximum and minimum values
|
Grade of Fuel |
Average heat change per litre |
Max heat change per Litre |
Min heat change per Litre |
|
Standard |
11066.8 |
11680 |
10512 |
|
Advanced |
9957.2 |
10366 |
9198 |
|
Premium |
7358.4 |
8176 |
6570 |
|
Diesel |
5594.4 |
6384 |
5040 |
Conclusion
Premium gas which has a higher octane rating will be more efficient than standard gas. Premium gas contains less ethanol, which has short chains, so has more longer chains which release more energy. This hypothesis is incorrect since the efficiency of an engine is not measured by the fuel used. There are many other factors that make a engine run efficiently instead of the fuel, such as compression ratio, displacement, speed of engine size of cylinder and many other factors. The experimental data shows that the highest energy change per litre occurs in standard fuel and there is a downward trend towards diesel. This is clearly shown in graph 1. The amount of ethanol in the fuels may be affecting the comparison of each grade of fuel. It’s possible to calculate the energy coming from ethanol and then compare the grades of fuel based on their octane rating. This was not what was expected as premium gas was expected to be the most efficient since it has the highest amount of longer carbon chains. The graph represents a linear slope which is within the errors, so this graph shows my accuracy and precision with the lab.
As i proceed the lab i learned that the fume hood was causing my flame to become unstable and shift at random. This is a big error since the flame did not constantly heat up the erlenmeyer flask through the allotted time. Another source of error could be the wick, since it had to be left soaking the fuel to be fully absorbed into the fibers to allow proper combustion to occur. The 50 ml of fuel was then poured into the spirit burner to after the wick was fully soaked to make sure there was no extra fuel being soaked by the wick. Furthermore, if deionized water was used in this experiment the sources of error could be further reduced since the chances of impurities in the deionized water would be very low. Also, I would use my time more efficiently by preparing the next set of trials as the experiment was taking place since 5 minutes of standing around and doing nothing would be a waste of time, when i could be filling up my beaker with water to carry out my next results. If i was given the chance to carry out my experiment one again I would make some minor adjustments, for example, i would use erlenmeyer flasks instead of one, since it took a long time to clean and fill up. I could quickly switch the heated erlimyer flask for the fresh one to carry out the rest of my experiment and before i walk away to clean the used flask i would start my next trial.
References
- https://www.ocean.washington.edu/courses/envir215/energynumbers.pdf
- https://www.quora.com/How-much-energy-is-released-by-burning-1-litre-of-petrol
- https://iet.jrc.ec.europa.eu/about-jec/sites/iet.jrc.ec.europa.eu.about-jec/files/documents/report_2014/wtt_appendix_1_v4a.pdf
- https://www.rapidtables.com/convert/energy/Joule_to_Calorie.html
- https://auto.howstuffworks.com/fuel-efficiency/fuel-consumption/gas-price.htm
- https://www.bobistheoilguy.com/forums/ubbthreads.php?ubb=showflat&Number=272881
- https://afdc.energy.gov/fuels/fuel_comparison_chart.pdf
- http://chemed.chem.purdue.edu/genchem/topicreview/bp/1organic/coal.html
- https://www.pacelabs.com/environmental-services/energy-services-forensics/forensics-101-a-primer/identifying-hydrocarbons.html
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


