Ionic Bonding
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1403 words | ✅ Published: 23 Sep 2019 |
Sodium chloride, NaCl, is made up of sodium and chlorine atoms bonded together. Sodium is a metal found on the left-hand side of the periodic table. Chlorine is a halogen (non-metal) found on the right-hand side of the periodic table.
According to the octet rule, it is preferable for atoms to have full noble gas configuration as this is the most stable atomic configuration with a lower energy. Atoms can achieve noble gas configuration by either sharing pairs of electrons, forming covalent bonds, or donating and accepting electrons to give positively and negatively charged ions, forming ionic bonds.
Ionic bonds tend to form when the outermost electrons of an atom of lower electronegativity have completely transferred to an electron of higher electronegativity. Electronegativity is an atom’s ability to attract electrons from another atom. Atoms with greater electronegativities have smaller atomic radii and atoms with smaller electronegativities have larger atomic radii. This is due to the attraction between the nucleus of one atom having electrostatic interactions with the valence electrons of another atom. The smaller the atom the more nucleic attraction and the larger the atom the more shielding and less nucleic attraction. Hence why ionic bonds form between sodium and chlorine atoms to form sodium chloride.
Sodium, an atom of lower electronegativity, donates its valence electron to the chlorine atom, an atom of higher electronegativity, fulfilling both of their octets and forming sodium ions (Na+) and chloride ions (Cl–) in the process (Figure 1).
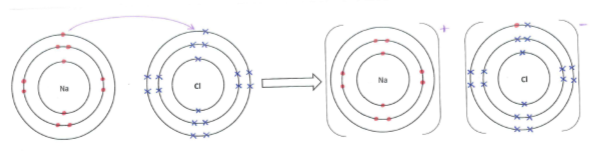
Figure 1: The donating of an electron from a sodium atom to a chlorine atom forming sodium and chlorine ions.
At the correct interatomic distance there is enough electrostatic attraction between the two particles to release enough energy to make the reaction feasible.
The oppositely charged ions of sodium chloride form a giant ionic lattice crystal structure. The electrostatic interactions between the sodium and chloride ions hold the structure together. Each ion is electrostatically attracted to ions of the opposite charge but electrostatically repelled to ions of the same charge. This means that the electrostatic interactions are non-directional and hence the physical structure of an ionic crystal lattice, including sodium chloride, is determined purely by spatial geometry.
The sodium ions and chloride ions in sodium chloride are known as being 6:6 co-ordinated (Figure 2) which means that each sodium ion is adjacent to 6 chloride ions and each chloride ion is adjacent to 6 sodium ions.
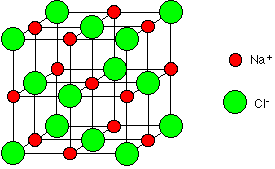
Figure 2: 6:6 co-ordinate structure of NaCl unit cell.
https://www.chemguide.co.uk/atoms/structures/ionicstruct.html
[Accessed 19 November 2018]
Sodium chloride is 6:6 co-ordinated because it is the most stable structure for the ions. Stability is achieved by releasing as much energy as possible. Electrostatic attractions between oppositely charged ions releases energy, so the more attractions formed the more energy is released and the greater the stability of the crystal. Therefore, a chloride ion forms as many attractions with sodium ions as it can before the chloride ion starts to touch other chloride ions and disrupt the stability of the crystal. Due to the size of the chloride ions and sodium ions 6 attractions can be formed.
It should be noted that the above model of the 6:6 co-ordinate structure of NaCl should not be assumed as the actual structure. It is merely a representation of the relative placement of ions in relationship to each other. From the above model we would presume that there is space in between each ion whereas in actuality there is no space between the ions at all. A better representation of the structure of NaCl can be found below (Figure 3).
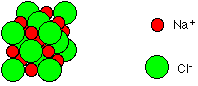
Figure 3: Space-filling diagram of NaCl
https://www.chemguide.co.uk/atoms/structures/ionicstruct.html
[Accessed 19 November 2018]
When discussing the crystal structure of sodium chloride, we must first understand that the crystal structure is made up of multiple repeating unit cells. It is useful to think of the ions being solid spheres when working out structures. In sodium chloride the ions form face-centred cubic packing cells which is made up of multiple layers. The first close packed layer is formed where each ion is surrounded by 6 other ions, the second layer sits in the gaps of the first layer (Figure 4) and the third layer sits in the empty spaces above the first layer (Figure 5).
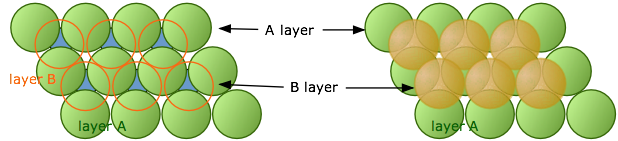
Figure 4: Layers A and B
http://www.chem1.com/acad/webtext/states/crystals-cubic.html
[Accessed 26 November 2018]
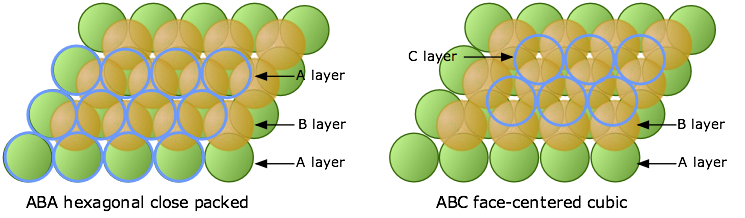
Figure 5: Third layer forming hexagonal close packing or face-centred cubic close packing
http://www.chem1.com/acad/webtext/states/crystals-cubic.html
[Accessed 26 November 2018]
Another type of packing called hexagonal close packing can be formed by placing the third layer directly above the first layer however this is not the case in sodium chloride. Face-centred cubic close packing forms an ABCABC layered structure whereas hexagonal close packing forms an ABABAB layered structure. The unit cell of sodium chloride can be thought of as two mutually interpenetrating slightly expanded face-centred cubic arrays of ions (Atkins and Paula, 2002).
In face-centred close packing there are two types of interstices (holes) formed: Octahedral interstices and tetrahedral interstices (Figure 6).
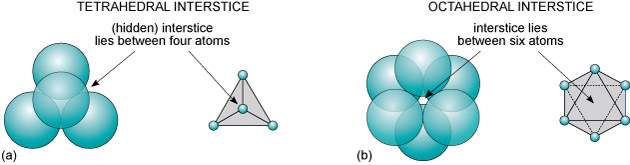
Figure 6: Interstices (vacant sites) between two close-packed planes of spheres: (a) tetrahedral interstice; (b) octahedral interstice.
https://www.open.edu/openlearn/ocw/mod/oucontent/view.php?printable=1&id=20276&extra=thumbnail_idp3150224
[Accessed 27 November 2018]
This is due to the different shaped holes formed by the arrangement of the chloride ions in the three different layers. The octahedral interstices are filled with the sodium ions and the tetrahedral interstices are empty.
Due to this very strong and ordered giant ionic crystal structure, sodium chloride is found as small cubic crystals (Figure 7) which can be transparent or white.

Figure 7: Sodium chloride crystals.
https://dkphoto.photoshelter.com/image/I0000lSaeq7GweyY
[Accessed 03 December 2018]
The physical properties of sodium chloride can be explained by understanding the bonding and structure of the crystals.
Sodium chloride has a very high melting point of 800.7 oC (Haynes, 2014-2015). This is due to the electrostatic attractions between the oppositely charged ions in sodium chloride together with the closely packed structure of the crystal means that a lot of energy is required to overcome these attractions and hence the melting point is very high. The size of the ions in the crystal also influence the melting point since the smaller the ions are, the closer they are together, and hence the stronger the electrostatic interactions making the melting point higher. For example, rubidium iodide has a slightly lower melting point than sodium chloride since the rubidium and iodide ions are slightly larger than the sodium and chloride ions. Ions with a greater charge will form stronger electrostatic attractions than ions with lower charges. For example, magnesium oxide which contains Mg2+ and O2- ions has a higher melting point than sodium chloride which contains Na+ ions and Cl– ions.
The crystals of sodium chloride are also very brittle (Figure 8).
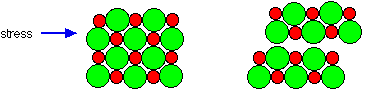
Figure 8: The movement of layers in a crystal due to stress explains the brittleness of sodium chloride
https://www.chemguide.co.uk/atoms/structures/ionicstruct.html
[Accessed 05 December 2018]
This is due to the interactions of ions when layers of the crystal shifted slightly due to force. If a layer is shifted in any direction then the ions of the same charge are brought together and repel each other, forcing the crystal apart.
Bibliography:
2015. The Sodium Chloride Structure [Online]. Structure World. Available: http://www.ilpi.com/inorganic/structures/nacl/index.html [Accessed 19 November 2018].
2016. The Octet Rule [Online]. Chemistry Libre Texts. Available: https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Electronic_Structure_of_Atoms_and_Molecules/Electronic_Configurations/The_Octet_Rule [Accessed 19 November 2018].
2018. Ionic and Covalent Bonds [Online]. Chemistry Libre Texts. Available: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Fundamentals/Ionic_and_Covalent_Bonds [Accessed 19 November 2018].
n.d.-a. Electronegativity [Online]. Dynamic Science. Available: http://www.dynamicscience.com.au/tester/solutions1/chemistry/electronegativity.htm [Accessed 27 November 2018].
Univerist of Kent (n.d.-b). Week 4: Ionic Bonding Study Guide.
ATKINS, P. & PAULA, J. D. (2002). Atkins’ Physical Chemistry.
CLARK, J. ChemGuide Ionic Structures [Online]. ChemGuide. Available: https://www.chemguide.co.uk/atoms/structures/ionicstruct.html [Accessed 19 November 2018].
HAYNES, W. M. (2014-2015). CRC Handbook of Chemistry and Physics, CRC Press LLC.
SHRIVER, D. F. & ATKINS, P. W. (1999). Inorganic Chemistry.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


