Identifying Macromolecules by Means of Colour Change
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1123 words | ✅ Published: 22 Jan 2018 |
By Marike Coetzee
B Dietetics II
1. Introduction
1.1 Aim
The purpose of this lab was to observe the colour changes (due to chemical reactions) indicators had on different macromolecules in food;1 such as starch, proteins and lipids; and then to identify the macromolecules found in an Unknown solution.
These known solutions contained different types of macromolecules which each reacted with at least one indicator solution in a unique way, which allowed us to identify the macromolecule based upon the presence or absence of a colour change. Water was also used as a control solution, as it showed a negative reaction with the indicator solutions.2
This experiment produced results which provided a clear understanding of the colour change that occurred when each known solution reacted with each indicator solution. When the colour changes associated with the Unknown solution were compared with those of the known solutions, it is possible to deduce which macromolecules are present in the Unknown solution
1.2 General background
The four types of macromolecules (organic compounds) found in all living organisms and substances are lipids, carbohydrates, proteins, and nucleic acids. Foods and nutrients, which consist of plants, animals or derivatives thereof; are a combination of these macromolecules.3 It is important to determine which macromolecules are found in food as macromolecules play an important role in nutrition.
The basis of this experiment is that the four types of macromolecules consist of different atoms and atom arrangements. Protein for example contains an NH group while carbohydrates contain a CHO group. This difference will cause the molecules to react differently with substances and enabling us to determine the compositions of different samples.4
ProteinCarbohydrate

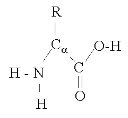
1.3 Focussed background
Negative and positive results for each indicator:1
Indicator |
Macromolecule |
Negative test |
Positive test |
| Iodine solution |
Complex carbohydrate |
Yellow |
Black |
|
Iodine |
Simple sugars |
Yellow |
Colourless |
|
Sudan III |
Lipid |
Light red |
Orange |
|
Biuret solution |
Protein |
Blue |
Violet |
*The intensity of the colours is an indication of the amount of the respective macromolecules found in the samples.
Water, protein, starch, lipid, vitamin C and an unknown sample were each treated with the different indicators solutions (iodine, Sudan III and Biuret & copper sulphate) to determine how that sample solution (and that type of macromolecule) would react with each specific indicator. The identity of the unknown solution can then be determined by comparing the reactions of the unknown solution with the reactions of the five known solutions.
2. Method and Materials
2.1 Materials/ Reagents
Apparatus used:
White paper
10ml test tubes
Pipette
Reagents:
WaterAscorbic AcidIodine solution
ProteinGlucoseSudan III
LipidUnknownBiuret & copper solution
2.2 Procedure:
- Set up three sets of seven clear, clean, 10ml test tubes and mark each set with the following: water, protein, starch, lipid, vitamin C and an unknown sample.
- Place 5 ml of each solution into each tube.
- Add one drop iodine to the first set of seven tubes, Sudan III to the second set and Biuret & copper sulphate to the last set of tubes.
- Place a white paper behind the tubes so any colour change can be easily observed
- Record the colour changes in the table below.
3. Results
|
Water |
Protein |
Starch |
Lipid |
Vitamin C |
Unknown |
|
|
Iodine |
yellow |
yellow |
colourless |
yellow drop |
colourless |
purple black |
|
Sudan III |
light red |
light red |
light red |
orange |
light red |
light red |
|
Biuret & Copper sulphate |
light blue |
dark purple |
light blue |
white with blue drop |
yellow |
light blue |
4. Discussion
- Interpretation of results
To create a control group, the different indicator solutions was added to water and used as a colour standard for a negative result. Any colour variation from the control group means a positive result.
The indicator solution that could best be used to show the presence of different starch molecules was Iodine as it turned from yellow to a dark/ purple black in the presence of starch.
Sudan III is the best indicator of lipids, since this solution turned orange, which is different than the controls red colour, and also only reacts with lipids and none of the other macromolecules.
To test for protein; a biuret & copper sulphate solution was used, since protein reacted with the biuret & copper sulphate to form a dark purple solution. This is considered a change since this solution (purple) is a different colour than the control solution (light blue).
Vitamin C has two indicator solutions that can be used to determine its identity: iodine and biuret & copper sulphate. The iodine indicator reacted with the vitamin C to produce a colourless solution, which can be contrasted with the yellow control, while the biuret and copper sulphate produced a yellow solution that differed from the light blue control.
- Broader implication of results
The Iodine solution turned yellow to colourless in the presence of simple carbohydrates (starch and Vitamin C) but it turned dark purple/black when it was added to the Unknown solution. From the focussed background information, it can be deduced that the Unknown sample contained a complex carbohydrate.
The Sudan III tested positive (changed from light red to orange) when added to a lipid but no colour change occurred when the indicator was added to the unknown. Therefore the unknown sample does not contain any lipids
Although the Biuret and Copper solution reacted differently for each of the protein, lipid and vitamin C; the solution stayed light blue when added to the starch and unknown. This proves again that the Unknown is a carbohydrate.
- Conclution
Due to the colour changes observed when the Unknown sample was treated with the different indicators, it can be deduced that the Unknown sample contained a complex carbohydrate, and none lipids or proteins.
The unknown solution, labelled Unknown, reacted with the iodine indicator to form a purple black solution. The only known solution which reacted with iodine to form a black solution was starch. Although these colours don’t match up perfectly, they are the closest match.
The difference in colour between the starch solution and the Unknown solution could be explained by a difference in concentration between the two solutions or by the difference in degree of complexity of the different starches. A factor that could have influence the difference in concentration is if the solutions weren’t both well shaken, since starch can settle out of solution.
5. References
- Yourscienceteacher.net [Internet]. Identifying macromolecules and Nutrients Lab background. Your Science teacher. [updated 2010; cited 2014-10-09]. Available from: http://www.cpet.ufl.edu/wp-content/uploads/2013/03/Identifying-Macromolecules-Lab.pdf
- Inky, EFA [Internet]. Identification of Macromolecules. Study Mode. [updated 2011-10-23; cited 2014-10-09]. Available from: http://www.studymode.com/essays/Identification-Of-Macromolecules-Lab-Report-810104.html
- nesscityschools.org [Internet]. Identifying macromolecules. Nesscity Schools [updated 2013; cited 2014-10-09]. Available from: http://www.nesscityschools.org/vimages/shared/vnews/stories/53f3996a455d1/Macromolecules%20Lab.pdf
- Smith JK [Internet]. Identifying macromolecules and proteins. Osborne Highschool [updated 2013; cited 2014-10-09]. Available from: http://osbornehighschool.typepad.com/files/biochemistry-lab-identifying-macromolecules2013s-with-post-lab-questions.doc
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


