Hazards of Hydrogen Peroxide in Hair Dye
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 4708 words | ✅ Published: 23 Sep 2019 |
Prologue:
After completing all the scientific units of this semester. I have learned plenty of new things such as: how to create a petition, how to cite a website in APA format, how to create a proposal letter, scientific terms, and even learned that gummy bears are reactive to liquid. More things I have learned from particular units as for space unit I learned a lot about astronauts and hazards they have to face. In Biology unit I learned more about one particular type of ecosystem. In Chemistry unit I learned how hydrogen peroxide is suitable for use in hair dye. Also, what are the hazards associated with this use? Lastly, in Electricity unit I learned how electrolysis separates elements of water naturally and even learned how to measure amps and volts of the circuit’s properties. I choose Chemistry and Biology to be connected because this was the only units that could connect with each other. Also, I am interested in biology and love to learn more about our different types of ecosystems. After completing all the units of science I think my strengths are in making lab reports and proposal letters because I think I communicate my ideas quite well by the usage of scientific terminology and my understanding. As a learner, I would improve on reducing the sources of errors of all my lab reports and would try to add more images in lab reports, so it is visually appealing.
Table of Contents:
Chapter 1: Hydrogen Peroxide
Chapter 2: First Chemistry unit connection to Biology unit
Chapter 3: Hydrogen Peroxide scientific structure
Chapter 4: Second Chemistry unit connection to Biology unit
Hydrogen Peroxide Information:
C1. I researched the chemical properties of hydrogen peroxide and how is it suitable for use in hair dye? Also what are the hazards associated with this use?
Hydrogen peroxide was discovered by Louis Jacque Thenard in the early 19th century. Hydrogen peroxide can also be called peroxide. It is a common ingredient in many hair dyes. Its chemical properties help to decolourize hair by reacting with the compound melanin, which is responsible for giving hair its colour. Peroxide changes melanin into a colourless compound, and the hair dye becomes a lighter colour.
Peroxide also helps to develop the dyeing compounds that are used to recolour hair. Caution must be taken when however, when using peroxide on hair. Several uses can damage the outer layer of hair portion, causing the hair to become brittle and break easily due to many chemicals in the substance. It can also cause itching, redness, and even skin blisters.
Some hazards utilized by the usage of hydrogen peroxide is that it is corrosive to skin, eyes, and any body part where it has been contacted. Also when hydrogen peroxide is contacted on skin and eyes it can cause a severe burn. Should avoid contact with hydrogen peroxide mist and should not be digested because it can seriously harm your body. Hydrogen peroxide usage in workplace should have eyewash stations and safety showers. In addition wear gloves, googles, and approved vapor respirator
First Aid guidelines if exposed to hydrogen peroxide:
● Inhalation: Go outside and breath in fresh air. If have hard time breathing, administer oxygen. If you cannot breath call the ambulance or contact the emergency number of the container.
● Eye Contact: immediately wash your eyes with warm water for approximately 15 minutes, and then call the ambulance.
● Skin Contact: Flush affected skin by a lot of warm luke water, then use unaffected soap and cream. Aslo remove any affected clothing, which has been extremely exposed to the hydrogen peroxide. Lastly call the medical help.
● Ingestion: Do not induce vomiting and loosen up tight and uncomfortable clothes. Immediately call medical help.
Always keep hydrogen peroxide products away from any sources of ignition, heat, and moisture, and children. Should always keep hydrogen peroxide away from acids, alkalis, flammable materials, catobalazing agents, metals, and etc. Always keep it in dry places and always seal it tightly. This chemical should be disposed accordingly by the label of the container.
How to dispose hydrogen peroxide chemical:
This amazing chemical act as a antiseptic for cuts, or wounds. This simple chemical can even kill microorganisms in food packaging materials. This chemical has to be properly disposed because then it may cause health problems to living organisms.
How to properly throw out food grade:
There is 35 percent concentration in the food grade. This percentage the food grade hydrogen is quite hazardous and needs to be took care of very cautiously. You need to follow these vital steps. Firstly you need to dilute it with the water, so it makes the chemical bonds weaken. Then you should decompose it with sodium after the mixing part. Always wear gloves, googles, apron, and etc to provide you with the best protection from those hazardous substances. Avoid getting splashed or contacted by the food grade because it can burn your skin. Also do not mix it in with substances like oil or combustibles.
Putting Hydrogen peroxide down the sink:
Many stores sell hydrogen peroxide in bottles with the concentration nearly to 1-4 percentage. Low concentration has no special procedure while disposing it. Instead you just need to drain the hydrogen peroxide this down the sink, and during this process you are able to clean the sink.
Classification of matter:
Mixtures is two or more substances combined in varying amounts. Physical means can separate mixture’s components.
Heterogeneous mixture is the particle composition which are different throughout each mixture. Each mixture has the different amount of particles of different substances. Some real life examples are milk (under a microscope), wood or concrete (at a close look), ice cubes in a drink, water and oil, and etcetera.
Homogeneous mixture is the particle composition. Each mixture type has a approximately the same number of particles of different substances. Such mixtures are also called solutions. Some examples are air, pure water, blood plasma, white vinegar, sugar water, corn oil, and etc.
Constant composition is a pure substance, where there are same particles. It cannot divide or separate physically.
Elements is composed of only one kind of atom (particle). It can’t separate into 2 or more substances by chemicals or physically. Examples are Iron (Fe), Copper (Cu), Oxygen (O2), and etcetera.
Compound are compounds which are made of just 1 kind of particle. Particles are made of several kinds of atom closely together in equalized proportion that does not differ. It could be divided into two or more substances by chemical (but not by the physical means). Examples are table salt (NaCl), sugar (C12 H12 O11), water (H2O), and etcetera.
Particle Theory:
Matter is something that have weight and which also utilizes its surrounding space. All matter is made of tiny particles. Particles are always moving, they move faster when they have more energy
(kinetic energy the energy of movement). Also the higher the temperature is the faster the particles move. The particles are attracted to each other. If the particles are closer to each other then the particle will be more attracted. Particles far apart have weak force but particles close together have strong force.
Density:
Density is the physical property of a substance. Density of matter image gas liquid solid.
Atom:
The atom is the smallest part of a property that keeps the element be itself. The atom is made out of smaller particles, which are called “subatomic particles”.
What atoms are made of:
An atom itself is made up of three tiny teeny kind of subatomic particles: protons, neutron, and electrons. The protons and neutron makes the atom’s center part, which is called nucleus and electrons fly over and above it creating a small cloud.
Physical property may be a attribute of substance which will be ascertained and measured while not dynamic the identity of the substance.
Chemical property has power of a material to change (react) and form new chemical ingredient. Example glow sticks are tubes containing different compounds that, when mixed, undergo a chemical reaction.
This produce light. The colour of the glow stick depends on the type of dye is in the stick. A large part of chemistry is based on reactions between substances, namely performing chemical reactions to synthesize new chemicals or products.
|
Description |
Example |
|
Reactivity with water paired compound could be a compound that reacts with water to form alkyne gas. The alkyne gas is flammable, that makes it helpful for generating light energy.This type of light energy was normal before battery operated and lights became available. Many caves still use this light source when they are underground in dark. |
|
|
Reactivity with O metallic element metal is extremely responsive with O, once attracted. The reaction causes layer of minerals to make on the bottom of the metallic element, that protects the metal from weathering. Additionally helps by keeping metallic element objects from chemical action within the exposed atmosphere. |
|
|
Reactivity with acids like hydrogen carbonate, or bicarbonate of soda, could be a compound that reacts with acids to form carbonic acid gas. Several instruction for food uses hydrogen carbonate as a result of the bubbles of carbonic acid gas that type facilitate to create batter and therefore the dough rise. |
|
|
Reactivity with the opposite pure substances Knowing however pure your substances react with one another provides the premise that permits chemist to develop new merchandise. |
|
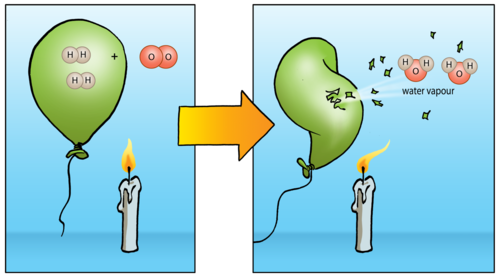
Hydrogen Peroxide chemical names are Oxydrol, Perhydrol, Superoxol, and etc. The molecular formula of Hydrogen Peroxide is the H2O2. The molecular weight is 34.014g/mol.
Physical description:
Hydrogen Peroxide is clear liquid. These liquid irritate the eyes and skin, when contacted on your body. Contact with most form of metals and their materials might cause damaging decomposition, particularly within the higher concentrations. Contact with inflammable materials might result an enormous fireplace. Can also result to fireside or heat decomposition and rupturing of the instrumentality. Oxide is additionally accustomed bleach textiles and pulp, in chemical producing and food process.
Hydrogen Peroxide is a crystalline solid at low temperatures. Has a slightly irritating odor. Used in the bleaching and removing the smell of many materials. Hydrogen Peroxide substitute for chlorine in water and sewage treatment. Hydrogen Peroxide physical properties are quite similar to the water, except it is about 40% denser than water. Also H2O2 is a very strong compound, which gives up one oxygen atom and forms water as a byproduct.
Colour
It is an almost pale blue colour in its pure state, but colourless in solution
Odour
Slightly sharp odor, similar to the nitric acid.
Taste
Slightly acidic and bitter in taste.
Boiling Point
150.2 degree Celsius
Melting Point
-0.43 degree celsius (decompose)
Density
1.45 g/cm3
Solubility
Soluble in cold water
Soluble in alcohol
Soluble in in an exceedingly kind of organic solvents like radical esters.
Insoluble in petroleum
Solubility in water
Miscible
Vapor pressure
6 mmHg (40 °C)
Acidity (pKa)
11.77
Viscosity
1.245 cP (20 °C)
Stability
Hydrogen peroxide may be a terribly unstable compound that breaks down promptly to create molecular chemical element and water.
The uses of Hydrogen Peroxide:
Antiseptic: Hydrogen Peroxide has been used for antiseptic for years, its properties can destroy bacteria. We use hydrogen peroxide for minor cuts, bruises, wounds, and etc. When applied on the wound it foams and fizz when contacted with blood, which helps us take out the germs and bacteria from the cut. Hydrogen Peroxide is very helpful in killing germs, and the growth of microorganism which produce sickness, illness, and viruses.
Bleaching: Hydrogen Peroxide is used for bleaching purposes due to its abilities of H2O2. When a large organic molecule has a double bond then this bond gets the ability to absorb sunlight. During an oxidizing reaction this organic bond breaks and destroys it pigment and removes the colour. Hydrogen Peroxide then is a great product for hair dyeing because its byproducts/elements (water and Oxygen) are not to harmful to anyone in society or environment.
Pollution controller: Hydrogen Peroxide is also used for environmental protection. It finds use in air pollution control where it is used to clean the air. It is also used to restore and recover sewage and industrial material.
Scientist and Career Pathway:
Scientist and Career Pathway that connect to my overall project is John Charles Polanyi and his occupation as Hungarian-Canadian chemist.
John Charles’s family made him move to Ontario because at that period of time his home in England was transformed into a bombing focused area during World War II. After few months later, he returned back to his homeland to finish his studies. Later again returned to Canada for his 1st analysis work with the National analysis Council. A Nuclear physicist coached him. Setting off his enthusiasm for taking a research and interest at the conditions and energy states of molecules. John Charles Polanyi career pathway is Hungarian-Canadian chemist. As a physical scientific expert, Polanyi’s examination centered around response elements: the parts of substance responses and the way they alter from one state into a completely another. His another analysis at the University of Toronto was on the subtleties of however chemical element hydrogen and chemical element chloride communicate to form acid. They quantify the sunshine transmitted amid the state changes to follow the right movement of particles and therefore the infant results of the response whereas it had been occurring. This examination prompted Polanyi getting the 1986 Nobel Prize. He has ascertained and studied the chemical properties of the chemical element. He has observed and studied the chemical properties of the hydrogen. He has also experimented the hydrogen and saw its mechanics of substance responses and how they change from one state into another when interacted with different elements.
This career pathway is important because chemist use different types of chemicals every day and perform chemical reactions to observe what its reaction would be. They study different type of chemical properties. Chemistry is very important as a result of everything you are doing is chemistry! Our body is created by chemicals. Every Matter is created by different chemicals,so it is very important to study chemistry because basically everything is made up of chemicals. This study then later on help us chemistry industry, who make products that are safe to use (mainly from chemical properties reaction). Chemist plays an vital role in our community because we get to know more information on healthy life, and our preservation on it. All students who are going to become medicinist are required to understand the concepts of chemistry unit before going to their universities. Chemist study and develop medicine, which gives us information on how to find cures for any illness or disease by having least amount of disadvantages on the sick person.To avoid pain, sickness, illness, or any bad effects of the world we need chemists, so they can provide us a happy and healthy life. This career connects to the biology unit because Chemical principles are vital in modern cell biology because all living cells are made up of chemicals and many chemical steps happens in many living organisms.
Sources:
● Chemistry Textbook
● Grade 9 academic chemistry sites: https://sites.google.com/pdsb.net/snc1dtroina2017/chemistry
● Hydrogen peroxide. (n.d.). Retrieved December 5, 2018, from https://pubchem.ncbi.nlm.nih.gov/compound/hydrogen_peroxide
● Hydrogen Peroxide Uses, Benefits, and Chemical Facts. (n.d). (2018, August 21) Retrieved December 5, 2018 from https://www.chemicalsafetyfacts.org/hydrogen-peroxide/
● NJ Health. (2008). Hydrogen peroxide substance fact sheet. Retrieved from
https://nj.gov/health/eoh/rtkweb/documents/fs/1015.pdf
● U.S. Department of health and Human services. (n.d.). Household Products Database – Health and Safety Information on Household Products. Retrieved from https://hpd.nlm.nih.gov/cgi-bin/household/search?queryx=7722-84-1 & tbl=Tbl Chemicals
B1.
Hydrogen peroxide is less likely to influence your wellbeing when utilized by its directions.
Hydrogen peroxide is Associate in Nursing oxidizing specialist that’s deeply receptive and when contacted with moistness, debases quickly to form water and O. T. At a amount of thirty fifth, it’s thought of to be irritating and corrosive to the attention and moderately irritating on the contact. However, the statements “Poison” and “Danger – Corrosive to eyes and skin” is required on the label. Also if inhaled 90% of concentration the user can even die due to its toxicity. Once acquainted with the earth hydrogen peroxide changes quickly to water and O.
Hydrogen peroxide is portable in terrestrial and aquatic ecosystems after applying.Soil particle can even naturally absorb it because of its short half-existence. After getting absorbed, it then gets drained to groundwater naturally. This chemical is harmful to oceanic life forms and plants.
Hydrogen peroxide turns into water and O by different surroundings, such as chemicals, algae, zooplankton, and heterotrophic bacteria. Bacteria is main reason of how O and water seperates. H202 decomposes depending on its chemical, biological, and physical surroundings.
In view of accessible information for the substance, hydrogen peroxide is poisonous to amphibian life forms whenever overdosed. The item can be considered as promptly biodegradable. Hydrogen peroxide is an extremely receptive and fleeting polar substance and no bioaccumulation is normal.
Some impact of human activities on the sustainability of terrestrial and/or aquatic ecosystems by using the chemical hydrogen peroxide:
Hydrogen Peroxide is famously known as a water cleanser in many terrestrial and aquatic ecosystems for the control of deaths of many fishes, who were suffering bacterial gill disease (BGD).
Some major human impacts on the sustainability of terrestrial and/or aquatic ecosystems by using the chemical hydrogen peroxide:
- Cure of external saprolegniasis in fish.
- Cure of bacterial pill disease on all water bodies (especially great at recovering the salmoids fishes population rate) .
- Cure of external columnaris disease on all water bodies.
- Cure on decreasing the level of Bacteria and germs -Peroxide is employed in cultivation to regulate external microorganism infections and infestations on fishes. Even widely used throughout the planet in human health for terrestrial and/or aquatic ecosystems.
Hydrogen peroxide is an effective in recovering dirty water, which is balancing the sustainability of terrestrial and/or aquatic ecosystems by the usage of the hydrogen peroxide chemical, without even producing negative impacts. If fishes just keep dying because of so many diseases and illnesses then our biodiversity would get worsen and we won’t have sustainable ecosystem because there are not equal amounts of species altogether, which cause the imbalance.
Sources:
● Ecology Textbook
● USGS. (n.d.). Retrieved January 7, 2019, from https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadEA/123
● Hydrogen Peroxide. (n.d.). Retrieved January 7, 2019, from
https://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=305&tid=55
C3.
The chemical structure of Hydrogen Peroxide:
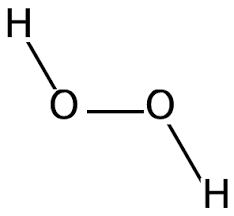
The Lewis structure of the hydrogen peroxide:
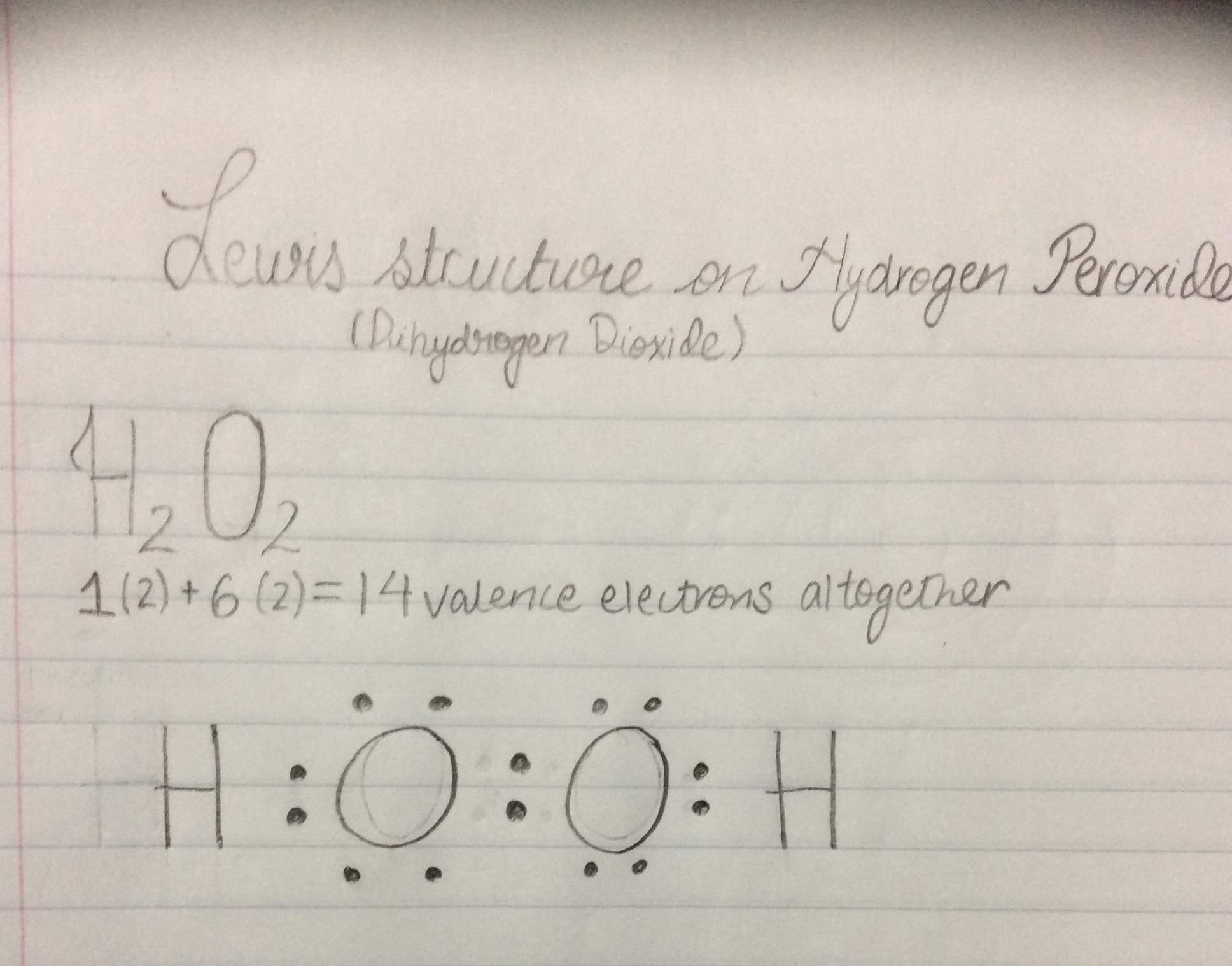
Hydrogen Peroxide valence electrons are quite stable because the oxygen has eight valence octet electrons, which tells us that they are stable in their outer orbitals.
Most of the elements are classified as either a metal or a non-metal, consistent with distinct materials. Metal is something which is solid and shiny. On the other hand non-metal is something that is not solid and shiny. Melataloid is something which have some qualities of metals and some of non-metals. The hydrogen peroxide chemical elements (hydrogen and oxygen) both are non-metal.
Hydrogen on the Periodic Table:
Oxygen on the Periodic Table:
B3.
Ecosystem dynamics is the investigation of how ecosystems change over time. Ecosystems are dynamic in nature, subject to normal micro and macro unsettling influences, both inside and outside. In our community, there are numerous occupations that keep things working easily. A nurse, engineer, junk collector, server, and rancher. Each person of a community plays a job. Even animals ecosystem requires each animal playing a different role in their ecosystem in order to keep ecosystem working easily. For an example, predators keep the population of their preys under control, insects fertilize blooms, and worms decompose leaf litter and dead animals. All animals help keep the ecosystem balanced. Ecological balance is a term describing how ecosystems are organized in a state of stability where species coexist with the other species and with their environment.
Here is some information on population:
● Population: Consists of all the people of identical kind, or species, that sleep in a given space.
● Community: Consist of all the populations that live in a given area.
● Species: Consist of the organisms so similar that they can mate and produce.
Population can change by four factors:
Natality: Number of births given in one year.
Mortality: Amount of deaths in one year.
Immigration: variety of people stepping into associate existing population.
Emigration: variety of people moving out of associate existing population.
Limiting factors: Ecosystem’s population is stopped by this factor. The factors are Light, temperature, food, predators, and diseases.
If an organism is getting favourable light, temperature, and chemical environment. Then it provides them with suitable habitat, good amount of food, ability to compete for resources, ability to defend a predator, and the ability to catch a disease or sickness.
If an organism is getting too little light, too high or low temperature, unfavourable chemical environment. Then it means that the organism does not have a suitable habitat, less amount of food, too many competitors, inability to hide or defend from a predator, and inability to catch disease or sickness. A healthy ecosystem is stated equilibrium because the population is stable and has been maintain at its carrying capacity. Carrying Capacity is the largest population of species that an ecosystem can support.
Here is some information on biotic and abiotic factors:
Biotic: Ecosystems living things. The biotic things includes trees, animals, and microorganisms.
Abiotic: Ecosystems non living things. The abiotic parts includes water, oxygen, light, nutrients, and soil.
Biotic factors is quite important for any ecosystem because biotic factors interact with our living things.
|
Biotic Interactions |
How it works |
|
Symbiosis |
Symbiosis is a interaction between members of two different animals type. Symbiosis is when two different animal types are in a symbiotic relationship because they can’t live without each other’s help. But when species live together by their choice it means it is a facultative symbiosis. Symbiotic organisms require other organisms help and if those animals organisms are important for our ecosystem, then symbiosis is necessary to keep the ecosystem functioning. |
|
Predation |
It is when one animal kill the other for their food source. The animal which is killed is called prey and the animals who killed it is called predator. This method is quite important in having a healthy ecosystem because predators eat prey for us, which are old, sick, injured, and etc. This also control the prey’s population and as well reduce diseases/sickness from spreading through ecosystems. |
|
Competition |
It is when two animals compete for same food source at the same time. This interaction proves which animal is most suited for this environment. If the animal is well suited for this ecosystem then it means they are able to survive, reproduce, and pass on their characteristics along their generations. Sadly, for animals who are not so well suited for the ecosystem, they eventually do not survive, reproduce, and pass their not so good characteristics through their generation. Even their population can also be removed. This process is important because it introduces new species and their improved characteristics. |
The Abiotic characteristics are the factors that living things survive from.
|
Abiotic Characteristics |
Why is it important |
|
Water |
All organisms need water to survive. Plants take up water through their roots. Some animals need water to regulate their temperature. Conjointly use water to induce obviate wastes.. Many organisms live in freshwater and saltwater ecosystem. Water is not only important for animals but it is also vital for humans as well in order to survive. |
|
Oxygen |
All organisms, including plants and animals need oxygen for their life processes. Aquatic organisms get oxygen from water as well. |
|
Light |
Plants and organisms need light for photosynthesis; a life process in which organisms produce their own food. |
|
Nutrients |
Plants and animals need nutrients, such as nitrogen and phosphorus in order to survive. |
|
Soil |
Gives minerals for trees and plants. |
As for my biology unit project I have created an urban proposal letter, which is all about my ideas and concepts of my future urban park. The chemistry unit connects to my concepts of the park by me having my fifth feature of the park being an area for people to come in and learn more about the sphere of our ecosystem, which explains the role of hydrogen peroxide chemical. Also learn why those spheres are important for all living organisms. I will also send them with flyers, posters, infographics, and etc, so they can learn more about spheres. This information will be also on social media account of the urban provincial park I would create on facebook, snapchat, instagram, twitter and ect. This way I can spread my message around very quickly. Lastly I will also create a special website for this park, which would have all the information.
Biosphere: Is the living surface of the Earth. This is what maintains and flow the energy through our ecosystem. However, sometimes it is also disrupted by humans. For instance, the O level lessen and the carbon dioxide level increase when humans cut down trees or burn fossil fuels.
Lithosphere: Earth’s solid surface. It is Earth’s solid, outer layer which contains the rigid crust and upper mantle. Also it runs 100 km down from the surface. Lithosphere is the upper crust of the Earth, which keeps its magma and temperature maintained, so that its mantle cycle keeps moving. Without this nothing could live on the Earth because the surface is what we live on and also it is what controls our temperature.
Hydrosphere:is that the surface lined in water, each salt and contemporary. Water cycle is moved through hydrosphere cycle. Firstly water is collects in clouds. Then falls to ground by the cause of rain or snow. Ths water collects/drain to rivers, lakes, and oceans. Then it evaporates into the atmosphere to start the cycle over again. Water is our source of life. It is what keeps us alive. The human body is made up of 60% of water ands this is an important indication of that water is a vital part of Earth. Drinking water helps us maintain organ health and allows the blood to maintain the consistency it requires to flow freely and transport oxygen and nutrients to every cell of the body. Drinking water also prevents us from getting horrible diseases. Drinking water is just not about being safe from diseases but also to maintain good health. Drinking water also helps get rid of toxin, which basically is a poisonous substance produced within living organisms and can cause diseases to a human body. Drinking water can get rid of toxins from physical reactions from outside sources of the consumption of contaminated water. Water is also essential in the process of production of food and agriculture. Lastly water is needed for santational purposes for all living organisms. Water also provides habitat to many animals. Basically all living things need water for survival.
Atmosphere: Is that the layer of air on top of the surface. Atmosphere contains water, carbon dioxide, ozone, and etc. Atmosphere is the only planet of the solar system that has atmosphere, this is what makes Earth livable. Atmosphere is just not only the layer of gases we breathe, it is also our blanket of protection, which supports from the blasts of heat and radiation from the sun. Atmosphere help keeps planet warm.
In that area I would tell how hydrogen peroxide is effective in cleaning and improving those affected and dirty spheres. Even pouring hydrogen peroxide down the drain helps improve water quality.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal