Gravimetric Analysis of a Chloride Salt
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1488 words | ✅ Published: 28 Nov 2017 |
- Rania Williams
- Nour Wehbe
Purpose
To discover the amount of chloride in a strange salt, in order to demonstrate regular methods used in gravimetric analysis
Theory
- This equation describes the reaction between silver ion and chloride which results in the product silver chloride.
Ag+ (aq) + Cl– (aq) → AgCl (s)
Silver nitrate is used to precipitate chloride because it gives the best results.
- AgCl Solubility in water:
Silver chloride’s solubility is very low; however the salt is still soluble to some degree. If precipitate is not complete, the results will be very low.
Ksp = 1.6 x 10-10
- Precipitation occurs in acid to greatly reduce any interference from acid ions. These ions form co-precipitates with silver in acid containing no charged ions. Due to co-precipitates the results would be higher. Also in order for precipitation to occur in acid there needs to be some excess of silver ion at the end of the reaction to reduce the chances of silver chloride becoming more soluble. Co-precipitation would result in higher results.
- Description: The precipitate is heated in order to coagulate it. When it coagulates it will become a clumpy colloidal like form. In this form it will become more difficult for the precipitate to penetrate the filter paper. If the coagulate did go through, the results would be lower. If nitric acid had not been added to the precipitate it would become more vulnerable in penetrating the filter paper. If this had happened the experiment would have to be done again as there would be no way to determine the percentage of chloride in the salt.
- Photodecomposition:
The equation for photodecomposition occurring in the air:
AgCl (s) → Ag (s) + ½Cl2(g)
When the silver chloride has dried and put into light it will decompose into chlorine and silver. If photodecomposition occurs in air, the results would be low, however if this decomposition occurred with excess silver ion in an aqueous solution there will be another reaction (3Cl2(g) + 9H2O (l) + 5Ag+ (aq) → 5AgCl (s) + ClO3–(aq) + 6H3O+ (l)), which will make the results high.
- How much precipitate is lost by washing with 100ml fresh water?
Ksp = [Ag][Cl]
x x = x2
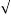 1.6 x 10-10 = 1.3 x 10-5
1.6 x 10-10 = 1.3 x 10-5
1.3 x 10-5= C/0.10 L
C =1.3 x 10-5x 143 mol/0.10 L C = 0.01859 mol/L
(0.01859)(0.10) = 0.001859
= 1.8×10-3 g
The precipitate is lost due to the solubility of it. The solubility of the precipitate is very low so not much would be lost, however this still would make the results lower.
- Ions that may co-precipitate with chloride ion:
When precipitation occurs quickly the chances of co-precipitation occurring greatly increases. Anions from some acids may co-precipitate with the chloride ion, forming co precipitates. These co precipitates will alter the results, making them CO32-, OH– and NO3–
Procedure
The code number of the unknown salt that was placed on the station was recorded. This sample was kept for the full duration of the experiment. Using the analytical balance, 0.1175g of the sample was weighed out by difference and placed in a 250ml beaker. The beaker was labeled to avoid confusion between partners.
The approximate volume of 0.1 M silver nitrate was calculated using the sample’s mass, 0.1175g. The mass of the sample was multiplied by the percentage concentration of the chloride then divided by 35.5. The result was then divided by 0.1. The result was converted into ml. 5 ml of excess was then added to the result, making the final result and approximate volume of silver nitrate added, 23ml.
In the 250ml beaker with the sample, 100ml of distilled water and 1ml of 6M nitric acid was added to the beaker. 23ml of 0.1M silver nitrate was measured out in a 25ml graduated cylinder then slowly poured into the 250ml beaker. The solution was placed on a hot plate then gently stirred. The solution was stirred until it became close to boiling.
In order to test for completeness the solution had a couple drops of silver nitrate poured into it to test that the entire chloride ion had been precipitated. The solution showed that it was complete. The 250ml beaker with the solution was then placed into the assigned drawer, to limit its light exposure.
Using a piece of soft tissue paper the crucible which had already cooled was weighed, it had a mass of 30.6707. The vacuum filtration arrangement was set up. The solution without the precipitate was slowly poured into the filter. 5ml of 0.1M nitric acid was used for washing the precipitate. After a couple washings the precipitate was also placed into the filter. A wash bottle was used to help any remaining precipitate out of the beaker. The precipitate was again washed with 0.1M nitric acid.
The crucible was then removed from the vacuum filtration arrangement. The leftover washings were disposed of. The crucible was washed once again in the vacuum filtration arrangement. The washing (mainly nitric acid) was taken to the T.A. for testing if the precipitation is complete by doing a washing with hydrogen chloride on the nitric acid. The first test showed completeness.
The crucible was again latched onto the vacuum filtration arrangement to be washed with 3ml of acetone. The acetone was handed to the T.A. for disposal. The crucible was given to the T.A. to put in the oven for drying of the precipitate. The oven had a starting temperature of 110 °C and after 30 minutes had a temperature of 119 °C.
The crucible was then cooled in the desiccator for 10 minutes then weighed with an analytical balance. The result was recorded.
Observations
|
Observation |
Description |
|
Physical description of sample |
Sample number:343 Sample had a light appearance, white. Very small grains. |
|
Tests for completeness of precipitation and for completeness of washing |
The result came back complete after 2 tests of completion and completion of washing. |
|
Final product |
The precipitate had coagulated and a light purple color on the outside. There was no specific shape to the precipitate, however it did look clumpy. |
Data tables
Sample masses
|
Mine (±0.0001g) |
Partner’s (±0.0001g) |
|
0.1175 |
0.1002 |
|
Sample Number: 343 |
|
Crucible masses
|
Item |
My Data (± 0.0001g) |
Partner’s Data (± 0.0001g) |
|
Empty clean crucible |
30.6707 |
30.8014 |
|
Crucible + dried sample mass |
30.9655 |
31.0320 |
|
Dried sample mass |
30.9655 – 30.6707 = 0.2948 |
31.0320 – 30.8014 = 0.2306 |
Approximate volume of the liquids and solutions used to was the sample
|
Approximate volume of: |
My Sample (± 0.2 mL) |
Partner’s Sample (± 0.2 mL) |
|
0.01M HNO3 |
5 |
5 |
|
Distilled H2O |
5 |
5 |
|
Acetone |
3 |
3 |
Temperature of Oven
|
Time |
Temperature (± 0.2 °C) |
|
Start |
110 |
|
End |
119 |
Crucible drying and cooling times:
|
Total time in oven (± 1 minute) |
Total time cooling (± 1 minute) |
|
30 minutes |
10 minutes |
Calculations
Amount of AgNO3 required (calculated amount + 5mL)
(0.1175)(0.55)/35.5/0.1
0.018204225 * 1000mL/1L = 18.204225
18.204225 + 5 = 23.20422525mL
23mL of AgNo3 needed
Percentage chloride in sample
|
Mine |
Partner’s |
|
0.2948 / 107.87 + 35.45 0.002056936 mol (0.002056936)(35.45) = 0.072918364g 0.072918364/0.1175 * 100 = 62.06% 62.05818255% |
0.2306/ 107.87 + 35.45 0.001608987 mol (0.001608987)(35.45) = 0.057038585g 0.057038585/0.1002 * 100 = 56.92% 56.92473551% |
|
Average = 62.05818255 + 56.92473551/2 = 59.49145903 = 59.49% |
|
Uncertainties
|
Mine |
Partner’s |
|
0.0002/0.1175g *100 + 0.0001/0.2948 *100 = 0.204134069 = 0.2041 |
0.0002/0.1002 *100 + 0.0001/0.2306 *100 = 0.242965933 = 0.2430 |
|
Average= 0.204134069 + 0.242965933/ 2 = 0.22355001 = 0.2236 |
|
Relative error
|
Mine |
Partner’s |
|
58.81 – 62.06 = -3.25 = 3.25 3.25/58.81 = 0.05526271 * 100 = 5.526271042 = 5.526% |
58.81 – 56.92 = 1.89 1.89/58.81 = 0.032137392 * 100 = 3.2137392 = 3.214% |
Relative spread of the percentage of chloride found
62.06% – 56.92% / 59.49%
= 0.086401076 * 10
= 0.86401076 ppt
= 0.8640 ppt
Discussion
My results were higher due to the photodecomposition of the precipitate that most likely occurred due to an excess of silver ion in the solution. This was a result of human error, as I waited for the precipitate to cool down I did not leave it out of light and failed to ensure that there was not an excess of silver ion in my solution. My results could also be higher due to any co-precipitates from anions such as these: CO32-, OH– and NO3– . The results could have also become higher due to not being washed properly. When washing the precipitate only with 3ml of acetone and 5ml of water this may have been possible. When compared with the actual result, my result was higher.
My partner’s results were lower than the real value due to some of her sample being lost during filtration. Sample being lost during filtration is almost unavoidable. Even though she may not have lost a lot of her sample, her initial salt mass was just 1.002g. Losing sample from a sample that was already so small contributed to her results being lower than the actual value. She also may have not allowed for complete precipitation of the chloride ion, resulting in lower results. During the heating of her solution her precipitate coagulated but there were stills some parts of the precipitate that were very tiny were susceptible of being loss the vacuum filtration. When compared with the actual result, my partner’s result was lower.
The average of my partner and I’s results were very close to the actual result, though the average of our results was still higher than the actual result.
Conclusion
The sample number for the unknown salt is 343. The average percentage of the chloride from two trials is 59.49%, whilst the actual percentage of chloride is 58.81%. The uncertainty for the percentage of chloride for my results was 0.2041 and 0.2430 for my partner. The precision of my results was 5.526%, whilst my partner’s was 3.214%. The accuracy of the results was 0.8640 ppt.
References
Books:
R.C.Burk, M.Azad, X.Sun, P.A. Wolff, Introductory Chemistry Laboratory Manual, Carleton University, Ottawa, 2014-15.
Websites:
Bishop, Mark. “Bases.” Bases. CHIRAL PUBLISHING COMPANY. 2013. Web. <http://preparatorychemistry.com/Bishop_Base_Identification.htm>.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


