Effect of Temperature on Ascorbic Acid Concentration in Broccoli
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2237 words | ✅ Published: 23 Sep 2019 |
How does temperature affect ascorbic acid concentration in broccoli, determined by redox titration with iodine?
1.0 Rationale:
When I was younger, my parents and grandparents would always talk to me about how we should eat more vegetables and fruits as they have the vitamins for me. What type of vitamins are in vegetables that are good for us? Well, turns out it is Ascorbic Acid, also known as Vitamin C, help our body plays a vital role in protecting the body from infection and disease. (Sifferlin A, 2013) It is not synthesised by the human body and therefore must be acquired from dietary sources – primarily fruits and vegetables. But there are many ways we can cook these vegetables or eating it fresh or frozen. So it got me thinking that does the cooking method or freshness of the plant affect the amount of ascorbic acid. So in this experiment I will compare the Ascorbic Acid content of broccoli when it is raw and then when it is cooked.
Research Question: How does temperature affect ascorbic acid concentration in broccoli, determined by redox titration with iodine.
2.0 Introduction:
There are two categories of vitamins, fat-soluble and water-soluble. (J. Fletcher, 2017) The fat-soluble vitamins are Vitamins A, D, E and K, which are stored in the body, are not easily lost from food when being cooked by water at different temperatures. (L. Bellows and R. Moore, 2018) Since these vitamins are stored in the body, they can be taken in one a large amount and will be used over a long time. Water-soluble vitamins are Vitamins C and B vitamins where they are not stored in the body, and are easily destroyed when cooking in water or by heat. In Table 2 (as seen below), it is a table talking about the different types of vegetables and the amount of ascorbic acid is in them.
|
Plant Source |
Amount of Vitamin C (mg / 100g) |
|
Plant Source |
Amount of Vitamin C (mg / 100g)
|
|
Rose Hip |
426 |
Spinach |
30 |
|
|
Red Pepper |
190 |
Potato |
20 |
|
|
Parsley |
130 |
Green Beans |
16 |
|
|
Broccoli |
90 |
Tomato |
10 |
|
|
Brussels sprouts |
80 |
Watermelon |
10 |
|
|
Elderberry |
60 |
Banana |
9 |
|
|
Strawberry |
60 |
Carrot |
9 |
|
|
Orange |
50 |
Apple |
6 |
|
|
Cantaloupe |
40 |
Lettuce |
4 |
|
|
Grapefruit |
30 |
Raisin |
2 |
Table 2: Amount of Ascorbic Acid in Various Plants (David A. Katz, 2013)
Vegetables consists of important vitamins and minerals. Unfortunately, the processing and preparation of vegetables like boiling or steaming them at different temperatures or freezing them can strip away much of their nutritional value. Ascorbic Acid can also be easily oxidized. In this experimental investigation, I will be testing the amount of ascorbic acid in broccoli to see if the temperature of broccoli affects it. And the word equation for this would be:
C6H8O6(aq) + I2(aq) —-> C6H6O6(aq) + 2H+(aq) + 2I -(aq)
Ascorbic Acid has the formula of C6H8O6 and it can be oxidised to form C6H6O6. The amount of Ascorbic Acid formed in a broccoli can therefore be calculated by titrating a known amount of the broccoli with an oxidising agent. In this experiment, Iodine, I2 (aq) is used as the oxidising agent. Therefore the equation for this experiment is formed below:
The starch solution is used as an indicator, and the broccoli solution will be titrated with the solution of iodine until one drop causes the blue-black colour.
3.0 Variables:
|
Independent Variables |
Dependent Variable |
Controlled Variable |
|
Temperature of the Broccoli: -20 , 0 , 20 , 40 , 80 , 100
|
Concentration of ascorbic acid in 10.0 ml aliquot of broccoli solution determined by redox titration with iodine. |
– Mass of broccoli (balance scale to weigh out the mases) different mases of broccoli will have different amounts of Ascorbic Acid. – Concentration Iodine |
Table 1: Table of Variables for this experiment
4.0 Safety Precautions:
– Goggles or safety glasses must be worn at all times in the lab
– The iodine solution is an irritant and will stain the skin and clothing.
– The iodine solution does give off a small amount of iodine vapours which are toxic when being inhaled, so gloves and goggles must be worn,
– Work in a well ventilated area. Keep the iodine solution covered.
– Dispose of all solutions in the waste containers or trash bins.
– Heat is also involved when rising the temperature of the broccoli, so hair must be tied back, goggles and gloves must also be worn
|
Materials Needed |
|
|
Equipment Needed: |
Solutions Needed: |
|
|
5.0 Methodology:
Preparation of Starch Solution:
1) Mix 1 g starch in 100 ml boiling water in a 250ml beaker
2) Boil for one minute while stirring.
3) Stir until completely dissolved (this solution will be cloudy).
4) Use 3ml for each trial of titration.
Preparation of fresh broccoli
5) Cut fresh broccoli into small pieces using a knife and weigh 3 x 100.00g using an electronic balance.
6) Then place the broccoli into the blender and blend for one minute.
7) Measure 50 ml of distilled water using 100 ml measuring cylinder and then add into the blender as well.
8) The material will then be blended using the highest speed for another 1 minute.
9) The mashed broccoli and water was transferred into a 500mL beaker and then strained with a cheesecloth into another 500mL beaker and then measured 10 ml using a measuring cylinder into a 250ml conical flask.
Preparation of frozen broccoli
10) Store broccoli for 2 (or 3) days.
11) After that take out the packaging of the broccoli.
12) Cut the frozen broccoli into small pieces with a knife and weigh 3 x 100.00g using an electric balance.
13) Repeat step 6 – 9.
Preparation of boiled broccoli
14) Take the packaging of the broccoli.
15) Cut the broccoli into small pieces with a knife and weigh 3 x 100.00g using an electric balance.
16) Start boiling the 150 ml of water in a 250 ml beaker.
17) Place broccoli pieces into the beaker and put the heating plate on highest heat.
18) Wait for the water to boil and take the broccoli pieces out.
19) Repeat step 6 – 9.
Redox Titration with Iodine
20) Approximately 3 ml of the starch solution was added to each conical flask containing the strained broccoli solution from the frozen fresh and boiled broccoli using a pipette.
21) Fill a burette with
iodine solution and record the initial volume reading.
22) Add iodine solution slowly into the conical flask while swirling the flask until the solution stays blue-black.
23) Record final volume on the burette.
*Modifications to method based on trial
I have also prepared a trial before conducting the full experiment and this then helped me to improve the method before collecting all the data. In my trial I used filter paper instead of cheesecloth to filter the broccoli from the solution as the filter paper did not filter everything between the broccoli bits and the solution with Ascorbic Acid. This then made me change a better filtration of the broccoli by using cheesecloth instead of filter paper. This then became part of a control experiment result for me to analyse in this Internal Investigation.
|
Temperature of Broccoli (
|
Trials |
Initial Reading from Burette ml (
|
Final Reading from Burette ml (
|
Volume of I2 added ml (
|
Average Volume of I2 added ml (
|
|
26 (Room Temperature, no heating and no freezing done) |
1 |
50
|
44.55
|
5.50 |
4.5 |
|
2 |
44.55
|
40.85
|
3.70 |
||
|
3 |
40.85
|
36.5
|
4.30 |
6.0 Analysis
Raw Data
|
Temperature of Broccoli (
|
Trials |
Initial Reading from Burette ml (
|
Final Reading from Burette ml (
|
Volume of I2 added ml (
|
Average Volume of I2 added ml (
|
|
-20
|
1 |
50.0
|
38.90
|
11.10 |
10.88 |
|
2 |
38.90
|
28.0
|
10.90 |
||
|
3 |
28.0
|
17.37
|
10.63 |
||
|
0
|
1 |
50.0
|
40.62
|
9.38 |
9.22 |
|
2 |
40.62
|
31.69
|
8.93 |
||
|
3 |
31.69
|
22.35
|
9.34 |
||
|
20 (heated) |
1 |
50
|
42.46
|
7.54 |
7.42 |
|
2 |
4
|
34.63
|
7.83 |
||
|
3 |
34.63
|
|
6.90 |
||
|
40
|
1 |
50
|
44.80
|
5.20 |
4.96 |
|
2 |
44.80
|
40.11
|
4.69 |
||
|
3 |
40.11
|
35.13
|
4.98 |
||
|
80
|
1 |
50.0
|
46.70
|
3.30 |
3.3 |
|
2 |
46.7
|
44.00
|
2.70 |
||
|
3 |
44.0
|
40.10
|
3.90 |
||
|
100
|
1 |
50.0
|
48.98
|
1.02 |
0.95 |
|
2 |
48.98
|
48.05
|
0.93 |
||
|
3 |
48.05
|
47.16
|
0.89 |
Table 1: Table of raw data collected from the experiment
Sample Calculations:
Here is an example on how the vitamin C concentration was calculated using data from the room temperature experiment.
STEP 1: Balanced Equation
C6H8O6(aq) + I2(aq) —-> C6H6O6(aq) + 2H+(aq) + 2I– (aq)
STEP 2: Calculate the average titre.
Average Titre = (Trial 1 + Tiral 2 + Trail 3 / Number of trials
Average Titre = (5.50+3.70+4.30) / 3
Average Titre = 4.50 cm3 (0.0045 dm3)
STEP 3: Calculate the moles of iodine required to react with the ascorbic acid in the 10ml aliquot.
n(I2) = 0.005 x 0.0045
= 2.25 x 10-5 mol
STEP 4: Calculate moles of ascorbic acid
Moles of Ascorbic Acid = Moles of Iodine
Refer to balanced equation above.
STEP 5: Concentration of Ascorbic Acid in 10ml (0.01dm3)
Concentration = mol/volume
Concentration = 2.25 x 10-5 mol/0.01
Concentration = 2.25×10-3 moldm-3
Processed Data:
|
Temperature (
|
Vitamin C in 10ml aliquot (moldm-3) |
|
-20 |
5.44×10-5 |
|
0 |
4.61×10-5 |
|
20 |
3.71×10-5 |
|
40 |
2.48×10-5 |
|
80 |
1.65×10-5 |
|
100 |
4.75×10-6 |
Table 2: Table of Processed data with calculations
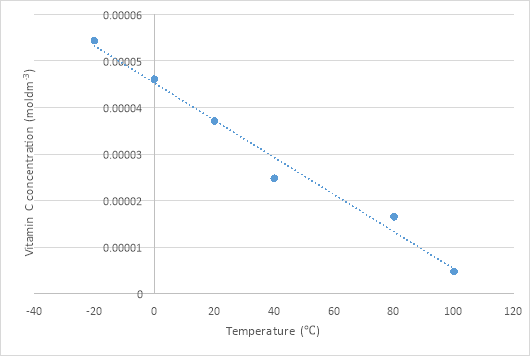
As temperature increases, so dos the Vitamin C concentration in a linear way. For example at -20
vitamin C concentration is at 5.44×10-5 moldm-3. Whereas at the temperature 100
, the Vitamin C Concentration is at 4.75×10-6 moldm-3. Ascorbic acid is highly sensitive to air, water, and temperature. About 25% of the ascorbic acid in vegetables can be lost when under intense heat for even a few minutes. According to my research, the same degree of loss will also occur when broccoli has been frozen. However, based on the experiment that had been carry out, the broccoli that was at -20
had a high concentration of Vitamin C. However, according to the graphs show, the broccoli at 100
has a lowest titre value and the broccoli that is at -20
has the highest titre value.
7.0 Evaluation:
Conclusion
In conclusion, the results show that the broccoli will have a higher titre value at different temperatures. This is probably caused by increased temperatures and lowered humidifies which accelerate oxidative, enzymatic and non-enzymatic activities associated with AA degradation. The lowest concentration of vitamin C is boil Broccoli at 150°C with vitamin C concentration is 14.37mg/ml. Heat decreases the vitamin C level. Vitamin C is damaged by heat by increasing its rate of oxidation.
Strengths and Limitations:
My graph is showing a clear trend and therefore I am confident that I have answer my research questions, all except 1 point, that are all close or on the trend line and they do share a correlation. The R2 has a number of 0.98132 and this means that the points on the graph does have a good correlation. During the experiment, I made sure that the controlled variables are all controlled well to ensure a fair experiment. Also, the results collected from this experiment has been consistent with research. There are also other methods and ways to determine ascorbic acid but I have chosen Starch and Iodine as it is easily accessible and the use of starch has a very strong end point.
However, the titrations repeated trials did not have a very close value between them as i hoped. This might be caused due to the misreading of the meniscus and thus I was not able to determine the end point accurately. I would use a whit paper in the future to make sure this limitation does not happen again. During the experiment, i have assumed that Ascorbic Acid is a water soluble molecule, that means that it would dissolve in water added into the blender but there is no way to be sure that all the Ascorbic Acid from the Broccoli would be in the water. So this is also another cause of error in the results taken from the experiment. Lastly., according to my research, that freezing of Broccoli would also cause the Vitamin C concentration to decrease but from what i have gotten, it did not. The possible reason for this is that the temperature was not properly controlled than the others and would need to be repeated again for more accurate results.
Further Research:
As a further research, taking into account that the results of this investigation it is concentration of the Ascorbic Acid in Broccoli will be lower when under heat, we can take into consideration on eating our vegetables raw so no Ascorbic Acid will be lost when consumed. As for freezing, the experiment needed to be repeated again as the data point was not like what I had research. According to my research, eating boiled and frozen is not beneficial to our health as there will be loss of Ascorbic Acid in the broccoli.
Bibliography:
- Bellows L. and Moore R. (2018) Fat-Soluble Vitamins: A, D, E, and K – 9.315. Retrieved from 22 January 2019, from extension.colostate.edu/topic-areas/nutrition-food-safety-health/fat-soluble-vitamins-a-d-e-and-k-9-315/.
- Fletcher, J. (2017) Fat-Soluble Vitamins: Types, Function, and Sources. Retrieved on 22 January 2019, from Medical News Today, MediLexicon International, www.medicalnewstoday.com/articles/320310.php.
- Katz D.A. (2013) Determination of Vitamin C in Foods [PDF file]. Retrieved on 22 January 2019, from http://www.chymist.com/Determination%20of%20Vitamin%20C%20in%20Foods.pdf
- Royal Society of Chemistry (n.d.) Multiple Resources Can Be Used To Talk About: Vitamin C [PDF file]. Retrieved on 22 January 2019, from http://www.rsc.org/learn-chemistry/collections/chemistry-in-health/content/Vitamin_C.pdf
- Sifferlin A. (2013) Broccoli Could Help Prevent Arthritis. Retrieved on 22 January 2019, from https://www.cnn.com/2013/08/28/health/broccoli-prevent-arthritis-time/index.html
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


