Dolutegravir Drug for Virologic Suppression
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2003 words | ✅ Published: 24 Jan 2018 |
Graphical abstract:

Dolutigravir, second generation integrase inhibitor: A new hope for HIV patients
- Geeta Yadav
Mesra, Ranchi
Abstract: Undeterred efforts have been made and will be made in future to make it possible for HIV-infected individuals to achieve the goals of virologic suppression and one more result of this rigrous exercise is dolutegravir drug. It is the recent integrase inhibitor approved by the US Food and Drug Administration (FDA) for use in the treatment-naïve, treatment-experienced, HIV-infected adults who have previously taken HIV therapy and also for children ages 12 years and older weighing at least 40 kilograms (kg) who are treatment-naïve or treatment-experienced but have not previously taken other integrase strand transfer inhibitors. This article has reviewed all the aspects of drug including the structural and functional analyses, in vitro activity, pharmacokinetics, drug-drug interactions, MOA, metabolism, excretion, dosing/ adverse effects and resistance profile of dolutegravir. Dolutegravir is a potent and generally well tolerated antiretroviral agent that may play an important role in the treatment of patients harboring resistance to other antiretrovirals.Some new combinations of drug with other antiretrovirals are also in pipeline which may hope to increase the immunologic response of the HIV patients.
Key words:Dolutegravir, antiretroviral, integrase inhibitor, HIV
Introduction
With the use of antiretrovirals with improved potency, tolerability, and resistance profiles, people with HIV are living longer and receiving longer-term care but even after so much advancement in therapy, they are struggling with an unknown fear of death [1, 2]. So, the need for new antiretroviral agents still continues to be substantial even after more than 20 years into the era of antiretroviral therapy, which have better tolerability, higher barriers to resistance, distinct resistance profiles, and fewer drug–drug interactions. These features of desiring drug have been inspiring the scientist all over the world to develop new agents that are not only focused on traditional targets but also on new novel therapeutic targets. The development of drugs targeting on critical steps in the life cycle of HIV-1 are drug classes that include HIV-1 reverse-transcriptase inhibitors (both nucleoside analogues and non-nucleoside inhibitors), HIV-1 protease inhibitors, and HIV-1 entry inhibitors (fusion inhibitors and CCR5 antagonists). The newest class of drugs in HIV treatment is the integrase inhibitor (INI) class.
Retroviral DNA Integration with the host DNA is an essential step in the life cycle of human immunodeficiency virus (HIV) [3], as shown in figure 1. This integration process is facilitated by the viral integrase (IN) enzyme which catalyzes the insertion of the viral DNA into the host genome in a multistep process. The process of HIV-1 integration occurs through 3 essential steps: formation of the preintegration viral DNA complex, 3’ processing and strand transfer [4]. HIV IN recognizes and binds specific sequences in the long terminal repeats (LTRs) of the viral retrotranscribed DNA in the cytoplasm. After DNA binding, IN cleaves GT dinucleotides from the 3’ termini of the linear cDNA in a process called 3’ processing .The processed viral DNA, as part of the preintegration complex, is then translocated into the nucleus, where IN inserts the viral DNA into the host chromosome by a process called strand transfer [4-6].

Figure 1 Schematic representation of HIV integration
Abbreviations: LTRs, long-term repeats; PIC, preintegration complex.
Integrase inhibitors (INIs) represent a class of drugs for the treatment of human immunodeficiency virus (HIV) infected individuals, blocking HIV genome transfer and integration into the host cell DNA [7]. In this category, first drug which got FDA approval was raltegravir (RAL) which have been found to be highly effective for the treatment of antiretroviral- naive and antiretroviral-experienced subjects and one more recent drug is elvitegravir (EVG) [8-12]. However, these first-generation INIs share common resistance pathways. During clinical studies of RAL, subjects with virologic failure and reduced RAL susceptibility typically are found to have virus with 1 of 3 signature mutational pathways (ie, N155H, Q148H/K/R, or Y143C/H/R) in the integrase gene [13]. So, continuing RAL treatment in these circumstances may lead to the addition of secondary mutations or pathways and N155H may evolve to Y143 or Q148 pathways [10]. In addition to this, EVG does not appear to have activity against RAL-resistant isolates and same case is with RAL [14-16]. Therefore, there is a need for an INI with a high barrier to resistance and activity in subjects with human immunodeficiency virus type. So, recent addition included in this category is Dolutegravir (DTG). This review article aims to covers all the aspects related to the dolutegravir which will help the scientists, academicians and common men to statisfy their knowledge pangs, like in vitro activity, pharmacokinetics, drug-drug interactions, MOA, metabolism, excretion, dosing/ adverse effects and resistance profile of dolutegravir as shown in figure 2, which exemplify methodology and evaluation of dolutegravir with the help of different information sources

Dolutegravir (DTG) discovered by a Shionogi and GlaxoSmithKline research collaboration, is a second generation novel HIV-1 integrase strand transfer inhibitor having activity against INI resistant viruses. In addition to it, also have favorable pharmacokinetic properties [17, 18]. It is indicated for use in combination with other antiretroviral agents for the treatment of HIV-1 in adults and children aged 12 years and older weighing at least 40 kg. It is available as a small, yellow, 50-mg tablet. Moreover, it can be taken with or without food and at any time of the day.
Structural and functional analyses of Dolutegravir (DTG)
Dolutegravir (DTG, S/GSK1349572) effectively inhibits HIV-1 IN variants which are resistant to the first-generation INIs. The structural basis for the increased potency of DTG resistant INIs is that it occupies almost the same physical space within the IN active site and make contacts with the β4-α2 loop of the catalytic core domain. Dolutegravir molecule has been divided into three main structural parts like tricyclic metal-chelating core, difluorophenyl ring and linker group which play a significant role in its binding to the protein as shown in figure 3. Tricyclic metal-chelating core binds to the intasome active site with the three coplanar oxygen atoms coordinated to Mg2+ cations The extended linker region connecting the metal chelating core and the halobenzyl group of DTG allows it to enter farther deeper into the pocket vacated by the displaced viral DNA base and to make more intimate contacts with viral DNA [19].

Figure 3 Structural and functional analysis of Dolutegravir
IN VITRO ACTIVITY
Dolutegravir has shown potent in vitro activity against both wild-type HIV and many INI-resistant mutants. It has potential for a higher genetic barrier to resistance. Dolutegravir has shown potent in vitro activity against HIV-1, with mean EC50 values of 0.5 nM (0.21 ng/mL) to 2.1 nM (0.85 ng/mL), IC50 of 2.7 nM and an IC90 of 2.0 nM in peripheral blood mononuclear cells (PBMC) and MT-4 cells. It also shows activity against HIV-2 virus with EC50 of 0.09 nM to 0.61 nM in PBMC assays. Cellular toxicity is also in the micromolar range for a variety of cell types, indicating that the observed antiviral effect of S/GSK1349572 are not due to cytotoxicity. S/GSK1349572 shows potency against all integrase- resistant single mutants with an FC as high as 3.6-fold. In the presence of S/GSK1349572 no virus with high resistance to S/GSK1349572 was observed with 32 nM or higher concentrations of S/GSK1349572 in the culture medium.
In vitro experimental studies reported that dolutegravir does not cause toxicity when used in combination, but had a synergistic effect with nevirapine, efavirenz, abacavir, stavudine, lopinavir, amprenavir, and enfuvirtide, as well as an additive effect when only used in combination with maraviroc. Efficacy of dolutegravir is also not affected on exposure to the adefovir and ribavirin [20].
Pharmacokinetics
Dolutegravir has a favourable pharmacokinetic profile without requirement of boosters and its terminal half-life is approximately 13–15 h [21, 22]. AUC0–24h and Cmax values are slightly less than the dose in the range of 2–50mg following single and multiple doses. One noteable change is the nonlinearness in Cmax and AUC with the increase in dose, So, a twice-daily 50mg regimen has been evaluated in the phase 3 ARV-experienced clinical trial rather than a once-daily 100mg dose [22-24]. The geometric mean steady-state concentration at the end of the dosing interval (Ctau) for a 50 mg dose was reported to be 1.6 μg/mL, which was approximately 25-fold higher than the protein-adjusted IC90 (0.064 μg/mL). A monotherapy study of, 10 days of dolutegravir 50mg daily dose in integrase inhibitor naïve HIV-1-infected adults demonstrated a 2.48 mean log10 reduction in HIV-1 RNA. This reduction was sustained for 4 days after discontinuation of dolutegravir only becoz of plasma concentrations which remained above the protein adjusted IC90. Overall, variability in exposure was minimal: 50 mg dosing to steady-state conditions achieved a geometric mean Cmax of 3.34 mg/ml (16% coefficient of variation), an AUC0–24h of 43.4 mg_h/ml (20% coefficient of variation), a t1/2 of 12.0 h (22% coefficient of variation) and a C24h of 0.83 mg/ml (26% coefficient of variation) [22]. A pediatric granule formulation of dolutegravir is currently in development. Preliminary data investigation reported that granules mixed in purified water have increased exposure compared with the tablet formulation with a geometric least-squares mean ratio (90% CI) for AUC0-inf of 1.57 (1.45–1.69) [23].
Drug–drug interactions
Dolutegravir pharmacokinetics has been evaluated in a single-dose crossover study for the effect of food and found that its absorption is modestly increased with food according to fat content [24]. Fat content affects the absorption of dolutegravir as noticed by the increased median Tmax from 2h to 3, 4, and 5h for low-fat, moderate-fat, and high-fat meals, respectively. Whereas dolutegravir AUC increased from 33 to 66% when administered with low-fat (300 kcal, 7% fat), moderate fat (600 kcal, 30% fat) and high fat food (870 kcal, 53% fat), respectively. [22, 24]. But these changes are not expected to affect safety or efficacy, So, dolutegravir can be dosed without regard to food. Dolutegravir causes drug-drug interactions with integrase inhibitors and some other drugs which is shown in Table 2.
Table 2. Dolutegravir (DTG) drug interaction with integrase inhibitors and other category drugs
|
S.No |
Interacting drug class |
Interacting drug |
Effect on dolutegravir |
|
1 |
Antiretrovirals NRTIs |
Tenofovir |
No significant effect observed[25] |
|
2 |
Antiretrovirals NNRTIs |
Efavirenz |
DTG AUC, Cmax, and Cmin decreased 57, 39, and 75% [26] |
|
Etravirine |
DTG AUC, Cmax, and Cmin decreased 70.6, 51.6, and 87.9%. [27] ETR/DRV/r administration results in 25, 11.8, and37.1% decreases in DTG AUC, Cmax, and Cmin |
||
|
ETR/LPV/r administration results in 11, 7, and 28% increases in DTG AUC, Cmax, and Cmin [27] |
|||
|
3 |
Antiretrovirals PIs |
Darunavir/r |
DTG AUC, Cmax, and Cmin decreased 22, 11, and 38% [28] |
|
Atazanavir |
DTG AUC, Cmax, and Cmin increased 91, 50, and 180% [29] |
||
|
Lopinavir/r |
No significant effect observed [28] |
||
|
Fosamprenavir |
DTG AUC, Cmax, and Cmin decreased 35, 24, and 49% [30] |
||
|
Tipranavir |
DTG AUC, Cmax, and Cmin decreased 59, 46, and 76% [26] |
||
|
4 |
Antituberculosis drugs |
Rifampin |
DTG AUC and Cmin increased 33 and 22% with DTG 50mg b.i.d.+ rifampin 600mg q.d. compared with DTG 50mg daily [31] |
|
Rifabutin |
DTG AUC and Cmin decreased 5 and 30%, Cmax increased 15 % [32] |
||
|
5 |
Acid-reducing agents- PPIs/H2 RA |
Omeprazole |
No significant effect observed [33] |
|
Antacids |
DTG AUC, Cmax, and Cmin decreased 73.6, 72.4, and 74.4% [33] |
DTG, Dolutegravir; ETR, Etravirine; EVG, Elvitegravir; LPV, Lopinavir; NNRTI, Non-nucleoside reverse transcriptase inhibitor; NRTI, Nucleos(t)ide reverse transcriptase inhibitor; PI, Protease Inhibitor; PPI, Proton pump inhibitor; r, Ritonavir; RAL, Raltegravir.
Mechanism of Action
Dolutegravir inhibits HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral deoxyribonucleic acid (DNA) integration which is essential for the HIV replication cycle as demonstrated in Figure 4. In this process, the integrase inhibitor chelate with the two Mg2+ ions in the integrase catalytic active site, unable the integrase enzyme to complete the strand transfer [21]. Inhibition of the integrase strand transfer reaction by DTG has been confirmed in studies with live virus, which demonstrated an accumulation of 2- long terminal repeat (2-LTR) circles in treated cells at DTG concentrations < 1,000-fold of those that caused cell toxicity [34,35].
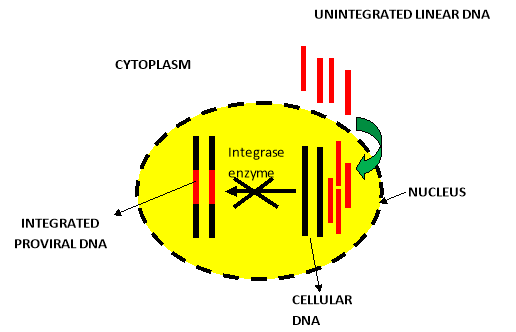


Figure 4. Mechanism of action of DTG
Metabolism/Excretion
Dolutegravir metabolism occurs through CYP3A4 (UGT1A1 glucuronidation) a major pathway while UGT1A3 and UGT1A9 are only minor pathways, which is catalysed by UDP-glucuronosyltransferase (UGT) 1A1 enzyme. In vitro studies reported that it is not a cytochrome P450 (CYP) inducer and neither an inhibitor. However, dolutegravir is an OCT2 inhibitor [21, 36]. Dolutegravir is also a substrate of UGT1A3, UGT1A9, BCRP, and P-gp in vitro [37]. It is the predominant circulating compound in plasma and the renal elimination of unchanged drug is extremely low (<1 % of the dose). Approximately 53% of a dose is recovered as DTG in feces and approximately 31 % is excreted in the urine primarily as DTG-glucuronide (DTG-Gluc) and other minor metabolites [38].

Figure 5. Metabolic pathway of dolutegravir
Dose/Adverse effects
Dolutegravir tablets are usually taken unboosted, orally and without regard to food [39]. Different dose combination studies with other drugs are reported to be performed to find the best combination with high resistance barrier as shown in table1. The most common adverse effects reported to be associated with dolutegravir Phase III SPRING-2 trial were nausea, headache, nasophryngitis, diarrhea and also a slight increase in creatinine level due to inhibition of creatinine secretion; however, dolutegravir had no effect on glomerular filtration rate [47, 48]. Some common drug -related adverse events were also notified during Phase III VIKING-3 trial in treatment-experienced subjects were diarrhea, nausea, and headache [49].
|
S.No |
Phase study |
Patients |
Dolutegravir vs other drug combinatons |
|
1 |
Phase III SPRING-2 Study |
Treatment naïve |
Dolutegravir 50 mg once daily versus raltegravir 400 mg twice daily, each in combination with either tenofovir DF/emtricitabine (Truvada) or abacavir/lamivudine (Epzicom) 40 |
|
2 |
Phase III SINGLE Study |
Treatment naïve |
Dolutegravir 50 mg in combination with abacavir/lamivudine (Epzicom) once daily versus tenofovir DF/emtricitabine/ efavirenz (Atripla) once daily41 |
|
3 |
Phase III SAILING Study |
Treatment experienced, integrase inhibitor-naïve |
Dolutegravir 50 mg once daily versus raltegravir 400 mg twice daily, each in combination with background therapy42 |
|
4 |
Phase III VIKING-3 Study |
Treatment-experienced with previous or current failure on raltegravir or elvitegravir |
Open-label dolutegravir 50 mg twice daily with current failing background regimen for 7 days, then with an optimized background regimen43 |
|
5 |
Phase III VIKING-4 Study |
Treatment-experienced with virus resistant to raltegravir and/ or elvitegravir at screening |
Dolutegravir 50 mg twice daily versus placebo , each in combination with current failing background regimen for 7 days, then with open-label dolutegravir 50 mg twice daily in combination with an optimized background regimen for both arms44 |
|
6 |
Combination under study |
A fixed-dose combination (FDC) tablet (dolutegravir 50 mg abacavir 600 mg/lamivudine 300 mg) and a dolutegravir pediatric granule45,46 |
Resistance
Dolutegravir (DTG) have been found to have a higher genetic barrier to resistance than raltegravir and elvitegravir [50]. Primary integrase resistance mutations associated with dolutegravir have not yet been identified. But viruses containing G140S, E138K, R148H, R263K, and G140S/Q148HRK mutations may show some level of resistance to dolutegravir. [50,39]. Raltegravir-resistant virus carrying a mutation at position Q148 had more reduced susceptibility to dolutegravir than isolates with other raltegravir mutations [51]. In vitro selection studies reported R263K mutation which commonly emerges in integrase in the presence of dolutegravir. R263K confers low-level resistance against dolutegravir and diminishes HIV DNA integration and viral fitness and no secondary mutation H51Y and E138K has been shown to compensate for the defects associated with the R263K primary resistance mutation against dolutegravir. All secondary mutations have a modest effect on resistance against this drug [52, 53].
Future of dolutegravir
ViiV Healthcare has requested US regulatory for the approval of a new single-tablet regimen (STR) containing dolutegravir, abacavir and lamivudine. A European regulatory application has also been submitted, according to the company. This combination, taken as separate pills, worked well in the aforementioned trials. If approved, the new co-formulation will offer the first one-pill, once-daily regimen that does not contain tenofovir/emtricitabine and could be particularly beneficial for people with, or at risk for, kidney disease or osteoporosis. Results from the primary analysis, presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) also reported that 90% of people taking dolutegravir and 83% taking darunavir/ritonavir achieved undetectable viral load in a ‘snapshot’ analysis, with dolutegravir meeting the criteria for statistical superiority. Based on these findings the researchers concluded that dolutegravir provide a potent and well-tolerated new option for first-line HIV treatment [54].
Conclusion
HIV-1 integrase is a unique target for antiretroviral therapy. Dolutegravir, a once-daily HIV strand integrase inhibitor currently approved for HIV-1 infected patients, provides at least equivalent antiviral efficacy and better tolerability compared with approved antiretroviral drugs. Efforts are ongoing for the approval of new single-tablet regimen (STR) containing dolutegravir, abacavir and lamivudine and also it would minimize the number of pills required for effective and acceptable antiretroviral treatment. Because of its unique mechanism of action, demonstrated virologic activity, resistance profile and tolerability, it is a significant advancement in HIV-1 therapeutics which will help HIV patients in long run.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


