Diketo Analogues and Their Significance
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1201 words | ✅ Published: 26 Jan 2018 |
INTRODUCTION
Medicinal chemistry is the branch of science, which has remarkable value for synthesis of novel drugs with intense therapeutic activity. It concerns with discovery, development, identification and interpretation of mode of action of biologically active compounds at molecular level.
The molecular biological revolution and progressive mapping of human ‘genome’ have created a new biochemical and biostructural ‘world order.’1
These developments have provided new challenges and opportunities for drug research in general and drug design in particular. Pure organic compounds, natural or synthetic products are the chief source of agents for the cure, the mitigation or the prevention of disease today. The major objectives of the medicinal chemists are transformation of pathobiochemical and physiological data into a ‘chemical language’ with the aim of designing molecules interacting specifically with the derailed or degenerating processes in the diseased organisms.
The development of chemotherapy during past 60 years constitute one of most important therapeutic advances in history of medicine and antimicrobial drugs are the greatest contribution of present century to therapeutics2. Potential therapeutic targets are being disclosed with increasing frequency and the exponential growth will continue during the next decades.
In this situation there is a need for rapid and effective target validation and for accelerated lead discovery procedures. Organic chemists are increasingly directing their attention towards synthetic aspects of biomolecules and biologically active compounds, biosynthesized by plants and animals.
Many important biochemical compounds and drugs of natural origin contain heterocyclic ring structures. Many of them are employed in treatment of many infectious diseases due to their specific activity, but their use in treatment is attributed to their inherent toxicity to various pathogens.
DIKETO ANALOGUES AND THEIR SIGNIFICANCE
Antibacterial
The quinolones3 are well known synthetic antibacterial agents with di keto moiety some examples are Ciprofloxacin (1) & Norfloxacin (2).

Boteva4 et al synthesized some Halogen Substituted 4,5-dibenzoyl-1-phenyl-1H-pyrrole-2,3-dione derivatives (2-6) & evaluated for Antibacterial Activity.

Anti HIV
The first report of a class of compounds that inhibit HIV integrase appeared in 1992. Aurin tricarboxylic acids and derivatives were determined to inhibit 3’ processing of viral cDNA with moderate iC50 values of 10-50micromolar.
Thus the 1st pharmacophore with integrase inhibitory activity was determined i.e. aromatic rings with multiple hydroxyl substituents positioned on same ring or present close together in a 3D space if rings stack on top of each other. Since then considerable amount of work has been carried out in developing potential inhibitors of integrase taking the above compounds as leads.
Presently there is only one FDA approved drug used as integrase inhibitor i.e. Raltegravir or Isentress, (7) approved in 2007. Elvitegravir (8) is another potential integrase inhibitor which is in phase III clinical trial5.

THIOPHENE ANALOGS AND THEIR SIGNIFICANCE
Thiophene (9) and its derivatives are an important class of heterocyclic compounds possessing broad biological activities, such as anti-inflammatory6, analgesic6, antioxidant7, antitubercular8, antidepressant9, sedative9, antiamoebic10, oral analgesic11, antimetabolite12, and antineoplastic properties13.

Antimicrobials
Thiophene analogues have been known as antibacterials. In the year 2007 Stephane et al14 reported the synthesis and antibacterial activity of arylbenzothiophenes (10) and diarylthiophenes. (11)

(10) (11)
In the year 2010 Kavitha P N et al15 reported the antimicrobial activity of 3- amino-2- mercapto-5,6,7,8-tetrahydrobenzo(b)thieno[2,3-d]pyrimidin-4(3H)-ones (12-17) by using B. subtilus, K. pneumonia and A. niger.

Desai Akshay et al16 reported the synthesis of 2-thiophene-2- ethylthioureido-4- morpholino-6-(aryl) ureido-s-triazines.(18-23) These analogs were evaluated for their antimicrobial activity using S. typhi, C. albicans.

Bhuiyan Md. Mosharef Hossain et al17 reported synthesis of 4-hydrazino-2- mehylthio-5-ethyl-6- methylthieno [2,3-d] pyrimidine (24) which is evaluated as antimicrobial agent by using B.cereus, V.cholerae, A.alternate.

(24)
Shiradkar M. et al18 reported synthesis of N-[3-(substituted)-7H- [1,2,4] triazolo [3,4-b] [1,3,4] thiadiazine / thiadiazol-4,5,6,7- tetrahydrobenzo[b] thiophenes (25-32) as good antimicrobial agents by using E.coli, S.aureus, A.nigar.

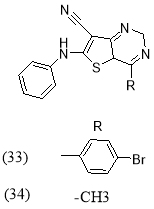
Ahmed M. M. et al19 reported synthesis of 4-(substituted)-7-cyano- 6-phenyl aminothieno [3,2-d] pyrimidins (33-34) and evaluated as good antimicrobial agents by using B.subtilis and St.aureus, compared with reference drug Amoxicillin.
The 6-methyl-2-phenyl-3-(substituted)-3H-thieno[3,2-d] pyrimidin-4-ones (35-38) has been reported by Chander Mohan et al20 and evaluated for antimicrobial activity by using B. subtilis, E.coli, P.aeruginosa compared with standard drug Ciprofloxacin.

Bhuiyan Md. Mosharef Hossain et al21 reported synthesis of thieno[3,2-e] imidazo[1,2-c] pyrimidin-2(3H)ones (39) which was evaluated for antimicrobial activity against B. cereus, S.typhi and A.alternatacompare to reference drugs Ampicillin with Nystatin.

(39)
Shetty Nitin kumar et al22 reported synthesis of 8,9,10,11-tetrahydro[1]benzothieno[3,2-e] [1,2,4]triazolo [1,5- c]pyrimidine -8-ones (40-42) and evaluated for antibacterial activity against B. subtilis comparable to ampicilin.

References :
- Larsen PK, Liljefors T, Madsen U, editors. Text book of drug design and discovery. 3rd ed. London: Taylor & Francis; 2002.
- Rang HP, Dale MM, Ritter JM Pharmacology, 4th edition Churchill Livingstone, Edinburgh, 1999: 648
- Wilson & Gisvold, Textbook of Organic Medicinal and Pharmaceutical Chemistry, 10th edition, Lippincott publication Philadelpia 196-203.
- A.A Boteva, O. P. Krasnykh, S.S.Dubrovina, M. I. Vakhrin, E.B. Babushkina, T.F. Odegova, I.V. Solova, Pharm Chem J , 2008, 42 (8), 12-15.
- Vincenzo Summa AlessiaPetrocchi, Fabio Bonelli, BenedettaCrescenzi, Monica Donghi, Marco Ferrara, Fabrizio Fiore, Cristina Gardelli, Odalys Gonzalez Paz, Daria J. Hazuda, Philip Jones, Olaf Kinzel, Ralph Laufer, Edith Monteagudo, Ester Muraglia, EmanuelaNizi, Federica Orvieto, Paola Pace, Giovanna Pescatore, Rita Scarpelli, Kara Stillmock, Marc V. Witmer, and Michael Rowley, J. Med. Chem. 2008, 51, 5843–5855.
- F.M. Moghaddam, H.Z. Boinee, An efficient and facile one-step synthesis of highly substituted thiophenes, Tetrahedron 60 (2004) 6085-6089.
- K.I. Molvi, M. Mansuri, V. Sudarsanam, et al., Synthesis, anti-inflammatory, analgesic and antioxidant activities of some tetrasubstituted thiophenes, J. Enzyme. Inhib. Med. Chem. 23 (2008) 829-838.
- M.K. Parai, G. Panda, V. Chaturvedi, Y.K. Manju, S. Sinha, Thiophene containing triarylmethanes as antitubercular agents, Bioorg. Med. Chem. Let. 18 (2008) 289-292.
- W. Wardakhan, O. Abdel-Salam, G. Elmegeed, Screening for antidepressant, sedative and analgesic activities of novel fused thiophene derivatives, Acta. Pharm. 58 (2008) 1-14.
- S. Sharma, F. Athar, M.R. Maurya, A. Azam, Copper(II) complexes with substituted thiosemicarbazones of thiophene-2-carboxaldehyde: synthesis, characterization and antiamoebic activity against E. histolytica, Eur. J. Med. Chem. 40 (2005) 1414-1419.
- O. F. William, Principles of Medicinal Chemistry, 3rd. ed., Lippincott Williams & Wilkins Publication, Philadelphia, 1989.
- A.A. Sagardoy, M. J. Gil, R. Villar, et al., Benzo[b]thiophene-6-carboxamide 1,1-dioxides: Inhibitors of human cancer cell growth at nanomolar concentrations, Bioorg. Med. Chem. 18 (2010) 5701-5707.
- A.A. Fadda, E. Abdel-Latif, R.E. El-Mekawy, Synthesis and molluscicidal activity of some new thiophene, thiadiazole and pyrazole derivatives, Eur. J. Med. Chem. 44 (2009) 1250-1256.
- Jeremie Fournier dit Chabert, Beatrice Marquez, Luc Neville, Lionel Joucla, Sylvie Broussous, Pascale Bouhours, Emilie David, Stephane Pellet Rostaing, Bernard Marquet,a Nicole Moreaub and Marc Lemairea, Synthesis and evaluation of new arylbenzo[b]thiophene and diarylthiophene derivatives as inhibitors of the NorA multidrug transporter of Staphylococcus aureus, Bioorganic & Medicinal Chemistry 15 (2007) 4482–4497.
- Kavitha PN, Vijayanthimala P, Saravanan J, Mohan S. Research Journal of Pharma- ceutical, Biological and Chemical Sciences, 2010; 1(2):124-130.
- Desai A, Mahajan HD, Ind Jour Chem, 2007; 46(B):1169-1173.
- Ahmed MM, Farha FM; Jordan Journal of Chem, 2008; 3(3):223-232.
- Shiradker M, Kale R. Ind Jour Chem, 2006; 46(B):1009-1013.
- Ahmed MM, Farha FM; Jordan Journal of Chem, 2008; 3(3):223-232.
- Mohan C, Bhargava G, Bedi PMS. J Life Sci, 2009; 1(2):97-101.
- Bhuiyan MH, Rahman KM. Acta Pharm., 2006; 56: 441-450.
- Nitinkumar SS, Lamani RS, Khazi IAM. Journal of Chem Sci, 2009; 121(3):301-307.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


