Determination of the End Point of the Acid Base Titration
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1161 words | ✅ Published: 18 Jan 2018 |
|
Marwan Mohsen Mohamed |
Table of Contents (Jump to)
pKa and Dissociation Equilibrium
Introduction
Acids and Bases
Every liquid we see will probably have either basic or acidic properties. Water can be a base and acid, it depends on the reaction you add with water. It can be a base in some reaction and an acid in some reactions. Also water can react with itself to form bases and acids but it happens in small quantities so it will not change your experiments.
2H2O –> H3O++ OH-
The hydrogen ion was transferred to form Hydronium ion. The negative and positive ions in water are equal and cancel each other. Most of water we drink from the tap has others ions in it. Those ions in solution make something basic or acidic. For example, in our Bodies, there are small compounds called amino acids and in fruits there something called citric acid.
According to Santé Arrhenius, in 1887, he came up with new definitions of acids and bases. He said when we mix water to molecules , they break down and gives a hydrogen ion and at another times it gives hydroxide. In general, a hydrogen positive ion is released, the acidic solution increases. When a hydroxide ion is released, the solution become base
For example
 HA +H2O H3O + + A–
HA +H2O H3O + + A–
Hydronium ion is formed and it is acid.
That hydrogen ion is the reason it is called an acid. Chemists use the word “dissociated” to describe the breakup of a compound
Properties of acid
- Acids taste sour
- Acids react strongly with metals (Zn + HCl)
- Strong Acids are dangerous and can burn your skin
Bases
- Bases are ionic compounds that break apart to form a negatively charged hydroxide ion (OH-) in water.
- The strength of a base is determined by the concentration of Hydroxide ions (OH-). The greater of the concentration of OH– ions the stronger the base.
Example: NaOH in water
 NaOH Na+ + OH–
NaOH Na+ + OH–
Strengths of Acids and Bases
Strong Acids and Weak Acids:
Strength of acid is related to ionization of acids in water. Some of the acids can ionize 100 % in water solutions; we call them “strong acids”. HCL are examples of strong acids.in other hand, some of the acids cannot ionize like strong acids. We call acids partially ionize in solutions “weak acid”. CH3COOH, HF, H2CO3 are examples of weak acid that partially ionize in solution
Strong and Weak Bases:
Bases ionize completely in solutions are called “strong bases”. NaOH and bases including OH- ion are strong bases. Bases that ionize partially in solutions are called “weak bases”. For example [ NH3]
Ionization of Water:
Water ionizes gives:
H2O(l) ↔ H+(aq) + OH–(aq)
In pure water concentrations of H+ and OH– ions are equal to each other and at 25°, they have concentration 1×10-7 M. then concentration of ion in pure water is too low, it is a bad electric conductor.
As in the case of pure water mediums having [H+] = [OH–] concentration are called neutral mediums. In water solutions multiplication of [H+] and [OH–] is constant and at 25 0C it is 1×10-14. This number is also called ionization constant of pure water.
If concentration of [H+] ions equal [OH-]= 10 -7M, then solution is neutral.
If concentration of [H+] ions > [OH-] or [H+] > 10 -7M and [OH-] < 10 -7 M, then solution is acidic.
If concentration of [OH-] ions > [H+] or [H+] < 10-7 M and [OH-] > 10-7 M, then solution is basic.
How to detect acid and Bases?
Scientists use something called pH scale to measure how basic or acidic the liquid is. Also there are many types of ions in a solution, pH focus on concentration of hydrogen ions and hydroxide ions. The scale measures values from 0 to 14. Distilled water is 7 in the middle. The strength of an acid or base in a solution is measured on a scale called a pH scale.
Any pH number greater than 7 is considered a base and any pH number less than 7 is considered an acid. 0 is the strongest acid and 14 is the strongest base.
The acid strength depends on the concentration of positive hydrogen ions in the solution. The greater and more hydrogen ions is the stronger acids likes Hydrochloric acid HCL and Sulphuric acid.

pH=-log[H+] and pOH=-log[OH-]
If 7>pH>0 acidic solution
If 14>pH>7 basic solution
If pH=7 neutral solution
pKa and Dissociation Equilibrium
1. pH
When acids is added to water, the pH scale decreases. The acidity of a solution is examined by the hydrogen ion concentration ([H+]), where pH provides a simple index for expressing the [H+] level., when pH is small which means that the smaller the number of pH , the stronger acid.
pH=-log10[H]
pKa and Dissociation Equilibrium
Strong acid , which they are dissociate in solution, and weak acids that partially dissociate in solution. When dissociation of strong acid happens, it gives a proton In which make the solution more acidic, However, weak acids have a dissociated state (A-) and undissociated state (AH) that appears according to the following dissociation equilibrium equation.
 AH A– + H+
AH A– + H+
. The definition of Ka is
Ka=
The brackets of the product to the brackets of the reactants
pKa was introduced as an index to express the acidity of weak acids, where pKa is defined as follows.
pKa= – log10Ka
Relation between Ka and Pka , it is inversely proportional so when ka is high which means storng acid which means pKa is low and vice versa
Equipment
- Burette

- Beaker

- Magnetic stirrer
- Ph meter

- Acid and Bases
- Pure water
Procedure
- Clean all equipment in order to get accurate conductivity
- Add some of NaOH into the receiving cup and then add slightly 1 ml of HCL and make sure you adding the receiving cup on the magnetic stirrer
- Repeat this steps to get the conductivity from volume 0 ml to 17 ml
- Get titration curve ,the differential curve and the end point
For CH3COOH + NaOH
We will make same steps and record the conductivity pH
Results and conclusion
NaOH + HCL
Results of volume of HCL and the conductivity
|
volume |
pH |
|
0 |
12.37 |
|
1 |
12.35 |
|
2 |
13.32 |
|
3 |
12.27 |
|
4 |
12.19 |
|
5 |
12.09 |
|
6 |
11.98 |
|
7 |
11.81 |
|
8 |
11.56 |
|
9 |
10.94 |
|
10 |
6.34 |
|
11 |
2.99 |
|
12 |
2.66 |
|
13 |
2.49 |
|
14 |
2.36 |
|
15 |
2.28 |
|
16 |
2.2 |
|
17 |
2.12 |
Ml pH
is clear that end point occurs at 10 ml of HCL which pH drops to 6.34
Titration curve
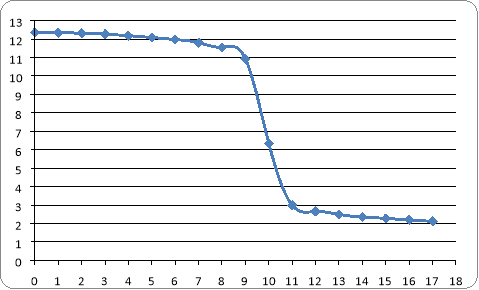
Differential curve

Ch3COOH +Naoh
|
volume |
pH |
|
0 |
3.24 |
|
1 |
2.62 |
|
2 |
3.95 |
|
3 |
4.20 |
|
4 |
4.40 |
|
5 |
4.60 |
|
6 |
4.78 |
|
7 |
4.99 |
|
8 |
5.24 |
|
9 |
5.64 |
|
10 |
9.43 |
|
11 |
11.19 |
|
12 |
11.51 |
|
13 |
11.72 |
|
14 |
11.85 |
|
15 |
11.95 |
|
16 |
12.05 |
It is clear that end point at 10 ml of Naoh the end point occur , the ph difference is very big
Titration curve

Differential curve

Bibliography
http://www.sciencebuddies.org/science-fair-projects/project_ideas/Chem_AcidsBasespHScale.shtml
http://www.elmhurst.edu/~chm/vchembook/184ph.html
http://lrs.ed.uiuc.edu/students/erlinger/water/background/ph.html
http://www.humboldtmfg.com/graduated_glass_beaker.html
http://www.shimadzu.com/an/hplc/support/lib/lctalk/29/29intro.html
http://www.princeton.edu/~achaney/tmve/wiki100k/docs/Acid_dissociation_constant.html
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


