Copper Recovery Methods From Metallurgical Waste
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1217 words | ✅ Published: 24 Jan 2018 |
REVIEW OF COPPER RECOVERY METHODS FROM METALLURGICAL WASTE
- Apurva Patel, Prof. Nimish Shah
Abstract: Copper is one of the most used metals in recent developments and demand of this red metal is increasing with passing of each day. Production of copper is 12 million tons per year and copper reserves are expected to run for 25 years with the estimated world copper reserves of 300 million tons. Recovery of copper from metallurgical waste is a trend that is being followed from beginning of industrial age and has many developments over a large time frame. Out of all the copper used in existing process, 2 million tons of copper is utilized with recycling of copper waste. Copper ore reserve contribution of India is limited and extended up to 2 percent of world reserve. We can say that copper has a large amount at our reserves but excavation is not as simple as it seems. Copper content in the raw mines is ranging from 0.5 to 1 percent. Even after recovery of copper there is large waste generated at the end of the process. Copper content in the waste is up to 0.3 percent at the discharge. Ultimately around hundred times of waste is generated for recovery of one part of copper. That pushes forward the need of recycling copper from metallurgical waste to cater the need of increasing copper demand. Copper recovery from high copper containing metallurgical wastes like brass industries are generally dealt with smelting process. In such case large amount of energy is utilized to just melt down all the material. This process has a limitation of copper content i.e. if copper content is low then all the energy is utilized in melting of undesired material. Demand for electroplating of copper has increased significantly. Low efficiency or improper process handling causes remarkably high copper content in waste discharge, which is over the range of discharge criteria of heavy metals. So to control the increasing price of metals and to limit the use of fresh copper, recycling must be done so the recovery from waste also gives the advantage of being in range of the allowable government legislations. Though these hazardous heavy metals in electroplating waste having concentration high enough to give harmful impacts to environment but convincingly low concentration that is not enough to recover these metals effectively. In this paper, overview of different methods for copper recovery is illustrated and justified the selection of different methods over different copper content of various sources.
Keywords— Copper extraction, Copper recovery, Electroplating, Recycling,
I. INTRODUCTION
Increasing demand of copper gives elevated chances for generating copper waste from different industries. There are thousands number of industries existing which includes utilization or processing of copper. In this paper, review of several most copper containing waste and most optimum copper recovery methods are described. Waste source is targeted which gives better possibilities of copper recovery and ease of operation. Several metallurgical source like; brass slag, copper converter slag, electroplating waste, bonze scrap and pickling solution is included in the study.
II. Copper recovery from Copper slag
There are different verities of slag produced from smelters for non-ferrous production. Major emphasis is given to copper slag as it has equal to or higher copper content compared to raw copper ore. Generation and utilization of copper slag has higher environment impacts compared to steel and iron slag as they contain remarkable quantity of heavy metals with higher solubility. Chemical composition of copper slag varies with different origins. Chemical composition is given as per Shen & Forssberg, 2003[1] in table 1.
TABLE I
Chemical composition of copper slag
|
Elements |
(Percentage) |
|
Cu |
0.6-3.2 |
|
Fe |
32.7-37.3 |
|
SiO2 |
32.5-37.3 |
|
Al2O3 |
2.4-4.0 |
|
CaO |
1.8-7.5 |
|
MgO |
1.6-4.0 |
|
S |
0.5-1.0 |
There are specific three methods to recover copper from copper slag; Floatation, Leaching and Roasting
- Floatation
Barnes (1993) [2] has given industrial floatation process at Mount Isa Mines Limited to recover copper from copper slag. Grinding operation is applied until obtain the granular size of 80%- 74 µm before floatation. Floatation is also feasible for magnetite present in the raw copper slag, so hydroxy ethyl cellulose is used in the process as a depressant of magnetite impurities. MIBC is used in the process as froather agent and sodium sec-butyl xanthate is used as a collector of copper from the waste. The result of this process gives concentrate grade copper with high percentage as 42.54%. Overall yield of such process is 82%. This experiment is observed for copper slag containing 3.7% copper. In this experiment most of Co is observed with floatation tail.
Mainly, copper slag floatation is somewhat similar with sulfide ore floatation because of the fact that only metallic copper and sulfide minerals from the copper slag can only be effectively floated. In other slag copper is usually observed under oxide state and Co and Ni are also in oxide state because of its homogeneous distribution in the slag. So the stated method will not be utilized effectively with Co, Ni and oxide copper state. Therefore the span for the floatation process is reduced in size as less quantity of Co, Ni must be present in the slag or copper must not be in the form of oxide.
- Leaching
Leaching is used with some leachants mainly hydrochloric acid, ferric chloride, ammonia, sulphuric acid etc. Basire at al anand at all [3]. In the initial era cyanide was also used but it was terminated because of its harmful effects to environment. Leaching is positively influenced by addition of H2O2, or leaching with Cl2/Cl– system, or pressure leaching. Figure 1 shows effect of H2O2 on leaching of copper based on the experiments of Base metal recovery [4]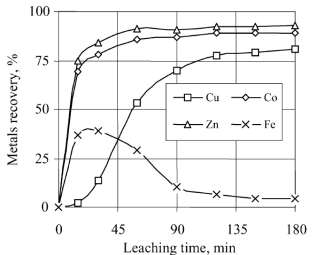
Figure 1. Kinetics of copper recovery
Graph showing metal recovery with highly oxidising agent like H2SO4 for copper slag. Experiment is carried out with 10% solid in solution and particle size less than 100 µm. Experiment is carried out at 70 oC and pH maintained at 2.5 with applied H2O2 at 35 L/(h.t)
Pressure leaching has broadly described by anand et al [4]. Study shows that with pressure leaching and use of dilute H2SO4 recovery of copper about 90% achieved from copper converter slag from the initial concentration of 4.03% Cu.
- Roasting
Roasting is actually one intermediate step which involves the process for converting the copper in desired form that can be easily separated from the raw material. After application of roasting, Leaching or floatation must be used to achieve desired separation. If we narrow down the process criteria then we can say a lot more specific term as sulfate roasting instead of roasting. In this process conversion of cupper cobalt ant nickel is taking place and transformed into more feasible soluble sulfates. Raw material is processed at 200-600 oC by addition of sulfide or sulfate agents. Then these soluble sulfates are dissolved in water and easily separated from slag. Some of the agents used in the process are, (NH3)SO4, H2SO4, H2S, pyrite etc. Sulfurization reactions are summarized as bellow.
Cu2O + H2S = Cu2S + H2O …(1)
2Cu + H2S + ½ O2 = Cu2S + H2O …(2)
Sulfides of copper are then easily converted to soluble copper sulfate with roasting at 600oC. Ziyadanogullari used this method to treat copper slag containing 2.4% copper. By sulfurization in closed system with 140oC for 1 hour and then heating and roasting with 600oC for 360 minutes gives better result for recovery of copper up to 99.2%.
SCHEMCON-20141 | Page
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


