Antimicrobial evaluation of Co (II) complex of 3-(3,4-Dihydroxyphenyl)-2-propenoic acid
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1808 words | ✅ Published: 26 Jan 2018 |
Antimicrobial activity of Co (II) complex of 3-(3,4-Dihydroxyphenyl)-2-propenoic acid and sonochemical synthesis of nanoscale mixed –ligand EDA coordination for preparation of CoCl2.6H2O anoparticle
Abstract
3-(3,4-Dihydroxyphenyl)-2-propenoic acid abbreviated as EDA was synthesized and characterized. Co (II) metal complex of this ligand prepared by reaction of chloride salt with EDA in dry acetonitrile. Phenolic compounds (a group of secondary metabolites) are widely distributed in plants and have shown to possess antimicrobial properties. Antibacterial activity was studied for ligand and its metal complex. This complex were tested for their antibacterial activity against Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pyogenes and Escherichia coli comparatively with that of free ligand. Differential response to phenolic compounds was observed among bacteria. Also this complex was synthesized in Nano-scale and was characterized by SEM, XDR (X- Ray powder diffraction). Antibacterial activity of complex and nano- material were studied and compared with each other.
Keywords: 3, 4-dihydroxy benzene acrylic acid, Cu (II) and Co (II) complexes, Antibacterial activity, nano- scale
Introduction
Acrylic acid or hydroxycinnamic acid compounds are widely distributed in the plants. They usually exist – number of them exist as free acids in nature [4, 5, 6].Much work has been realized by bioinorganic as well as medicinal chemists to launch the relationship between the metal ions and their complexes as antimicrobial agents [7-8]. Phenolic compounds are secondary plant metabolites and naturally present in almost all plant materials, including food products of plant origin. These compounds are thought to be an integral part of both human and animal diets [13]. The chemical structure of phenolic acids shows that they are simple phenols. Hydroxycinnamic acid is the major subgroup of phenolic compounds [11,12]. Hydroxycinnamates are phenylpropanoid metabolites and occur widely in plants[4],and plant products[2]. Hydroxycinnamates and their derivatives are bioactive plant food ingredients. The other natural ligand from plants such as alkaloids compound also can be used in synthesis of metal complex [1].
Nanophasic and nanostructured materials are attracting a great deal of attention because of their potential for achieving specific processes and selectivity, especially in biological and pharmaceutical applications [3]. Nanoparticles are made of natural or artificial polymers ranging [10].In particular, those conjugated with biological moieties have enormous potential in drug delivery and therapeutic applications. In fact, much progress has been achieved in the past ten years based on inorganic nanomaterials [9].
In this context we have undertaken the antimicrobial evaluation of Co (II) complex of 3-(3,4-Dihydroxyphenyl)-2-propenoic acid. For this purpose the in vitro susceptibility of tow gram positive bacteria (Staphylococcus aureus, Streptococcus pyogenes) and tow gram negative bacteria (Pseudomonas aeruginosa, Escherichia coli) to the synthesized compounds was investigated.
Materials and Methods
Synthesis of the metal complex; General Method
3-(3,4-Dihydroxyphenyl)-2-propenoic acid, cobalt chloride was Merck chemicals and was used without further purification. Organic solvents were reagent grade. Electronic spectra were recorded by Camspec UV–Visible spectrophotometer model Perkin Elmer Lambda 25. The IR spectra were recorded using FT-IR Bruker Tensor 27 spectrometer. 1H- NMR was recorded on a Bruker AVANCE DRX 500 spectrometer at 500 and 125MHz respectively. All the chemical shifts are quoted in ppm using the high-frequency positive convention; 1H -NMR spectra were referenced to external SiMe4. The percent composition of elements was obtained from the Microanalytical Laboratories, Department of Chemistry, University of tarbiyatmoallem, Tehran.
A solution of metal salt dissolved in acetonitrile added a dually to a stirred acetonitrile solution of the ligand (EDA), in the molar ratio 1:1 (metal: ligand). The reaction mixture was further stirred for 4-5h to ensure of the completing and precipitation of the formed complexes. Finally, the complexes dried in vacuum desiccators over anhydrous CaCl2.
Microorganisms and culture media
The following microorganisms were used in this study to test antimicrobial activity of complex. Escherichia coli, Staphylococcus aureus were kindly provided by (anistitue pastor ). The strains were maintained on PD3 agar at 26oC. For long- term storage, glycerol stocks of microorganisms were prepared in the corresponding growth media with a final glycerol concentration of 12%. The bacterial glycerol stocks were quickly frozen in liquid nitrogen and stored at -80oC.
In-vitro anti-bacterial activity
All methodology and steps were followed according to diffusion disk method. An inoculum of 0.5 McFarland standard (1.5*108 cfu/ml) was applied on Mueller Hintonagar (a depth of 4 mm in a petridish of 100 mm diameter) [14]. Maximum 6 discs were applied on each plate and they were incubated at 37 °C for 24 hours. Zone of inhibition was measured including the disc diameter (6mm).
Preparation of nanoparticles
Co nanocrystallites were prepared by the reaction of C9H8O4 with [Co (C9H7O4)] Cl2 in THF as solvent under ultrasound power. Then the suspension was irradiated for 1h with a high- density ultrasonic probe immersed directly into the solution under various conditions. A maltiwave ultrasonic generator ( sonicator – 3000: Italstructure MPD 3000). The samples were characterized with a scanning electron microscope (SEM) with gold coating.
Results and Discussions
Structural description of the complex
The reaction of Co(II) salt with the ligand, EDA, results in the formation of [ML] for M=Co (II). complex is quite stable and could be stored without any appreciable change. The EDA ligand and the [Co(C9H6O4)]Cl2.2H2O complex have 223-225°C and 195-198°C melting point respectively also complex is insoluble in common organic solvents, such as n-hexane and dichloromethane. However, that is soluble in DMSO, ethanol and DMF. its structure was characterized by elemental analysis, 1H-NMR and IR. Their elemental analyses are in accord with their proposed formula. The spectral data of the complexes have good relationship with the literature data.

Fig.1. Structure of the ligand, EDA.
Analysis of [ Co ( C9H6O4 )] Cl2.2H2O (EDACC): Dark Blue crystals; yield 86% .
elemental analyses, 1:1 metal to ligand stoicheiometry is assigned to all the chelates ( table 1).
Table1: Elemental analyses data on the caffeic acid and its Co (II) complex.
|
compound |
Elemental analyses %Found ( cal ) |
|
C H Co |
|
|
C9H8O4 |
78.28 5.68 – |
|
( 79.41) (5.88) – |
|
|
COL |
32.06 1.68 16. 25 |
(31.40) (1.74) (17.15)
1H-NMR: ( ppm DMSO, 500MHz): 5, 97-7.21 [5H, 2q, aroma]; 11.91 [1H, s, acid); 8.98-9,36[2H, s, alkene]. IR absorptions(cm-1 KBr): 1620 (C=C), 972 (=C-H), 1352 (C-O), 574 (Co-O) and 456 (Co-Cl).The electronic spectral data of the complex in acetonitrile are presented in table 2. there are one peak in spectrum of ligand which can be assigned to Π→Π* transition. The electronic complex shows a broad band at 680 nm attributable to the 4T1g ( F ) →4 A2g ( F) and the other one at 640- 550 nm attributable to the 4T1g ( F ) →4T1g ( P ) transition for Co
( II ) ion .
table 2: Electronic spectra of caffeic acid and its Co(II) complex in nm
|
Compound |
Interligand and charge transfer (CT) |
d-d |
|
solvent |
210 – |
– – |
|
COL |
240 280- 320 |
640-550 680 |
Raman shift (cm–1): 500  Co-O), 325 (Co-Cl), 975(C-H), 1618C=C), 1189C-O) (Fig.2.Left).
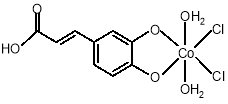
Fig.2. Structure of Co (II) complex with ligand, EDA.
In-vitro anti-bacterial activity
The mean diameters of microbial growth inhibited by different complexes are shown in Table 4. All complexes had antimicrobial activity. Inhibition zones larger than 5mm indicated that antimicrobial activity. The data obtained by the disk diffusion method showed that all complexes have antibacterial activity. Among the bacteria, Escherichia coli was the most sensitive bacteria both ligand and complex [Co(C9H7O4)]Cl2– Normal- scale had antibacterial effect on this bacteria , whereas only ligand C9H8O4 had antibacterial activity against Streptococcus pyogenes and Pseudomonas aeruginosa . Ligand C9H8O4 and complex [Co (C9H7O4)] Cl2( Normal- scale) had not any effect against Staphylococcus aureus.
Table 4. Zone of growth inhibition of the test compounds against the bacteria
|
Zone of growth inhibition (mm) |
||||
|
Complexes |
Pseudomonas aeruginosa |
Staphylococcus aureus |
Streptococcus pyogenes |
Escherichia coli |
|
C9H8O4 |
5mm |
– |
5mm |
5mm |
|
[Co(C9H7O4)]Cl2 Normal- scale |
– |
– |
– |
14mm |
|
[Co(C9H7O4)]Cl2 Nano- scale |
– |
4mm |
– |
– |
Complex [Co (C9H7O4)]Cl2 ( Normal– scale), had more antibacterial activity against Escherichia coli (14mm) . Complex [Co(C9H7O4)]Cl2 (Nano – scale), had less activity against Staphylococcus aureus (5mm). From these results it may be concluded that there is not any accordance between normal – scale and nano- scale from the antibacterial activity aspect. The antimicrobial activity of complexes demonstrated in this study can be added to the already known beneficial biological properties of these compounds to the human health.
Nanoparticle study
XRD pattern of mixture of caffeic acid andCoCl2 .6H2O prepared by the ultrasonic process is given in Fig. 4. The diffraction peaks accord with the amorphous crystal system.

Fig.4. The XRD pattern of the mixture of caffeic acid and 6H2O .CoCl2
The SEM micrographs of nanostructure are shown in Fig. 5. The nanoparticles show a low degree of crystalline with no defined peaks in the XRD pattern.



Fig. 5. SEM images of complex [Co (C9H7O4)]Cl2
The IR spectrum of Co (II) nanostructure (Fig.5) shows the absorption pesk at 574 – 648 are assigned to the ïµ(Co-O) modes, which confirms the formation of Co (II) nanostructure.
It has been reported that the negative charge on the cell surface of Gram-negative bacteria was higher than on Gram-positive bacteria (Chunget al., 2004). Due to a higher negative charge on cell surface, the
interaction between Gram-negative bacteria and nanoparticles was definitely stronger than that of Gram-positive bacteria. Moreover, results showed that Gram-positive bacteria were more sensitive to Co (II) nanoparticles.

Fig.6. IR Spectrum of complex [Co (C9H7O4)] Cl2
Acknowledgments
We gratefully acknowledge the financial support from the Research Council of Ardabil Islamic Azad University and many technical supports that provided by Ardabil University of. edical Sciences
References
[1] A.H. Osman, (Synthesis and characterization of cobalt (II) and nickel (II) complexes of some Schiff bases derived from 3-hydrazino-6-methyl [1, 2, 4] triazin-5(4H) one) Transition Met. Chem, 2006, 31, 35.
[2] Clifford, 1999 M.N. Clifford, Chlorogenic acids and other cinnamates: nature, occurrence and dietary burden, J. Sci. Food Agric. 79 (1999), pp. 362–372.
[3] Brigger, I., C. Dubernet, and P. Couvreur. 2002. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Delivery Rev. 54:631–651
[4] Herrmann, K. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci.Nutr. 1989, 28, 315-347
[5] Herrmann, K. Contents of principle plant phenols in fruits. Fluess. Obst 1992, 59, 66-70.
[6] Herrmann, K. Phenolcarboxylic acids in plant foods and their antioxidative activity. Gordian 1993, 93, 92-96.
[7] M.J. Seven and L. A. Johnson, “Metal Binding in Medicine”, Lippincott Co, Philadelphia, PA, 4ta Ed (1960).
[8] A. J. Crowe, Metal-Based Antitumor Drugs, Vol. 1, Freund, London, 1989, p. 103
[9] Gong, J., Hu, X., Wong, K., Zheng, Z., Yang, L., Lau,W., Du,R. Chitosan Nanostructures with Controllable Morphology Produced by a Nonaqueous Electrochemical Approach. Advanced materials. 2008, 20, 2111–2115.
[10] Kreuter, J. (2001). Nanoparticulate systems for brain delivery of drugs. Advanced Drug Delivery Reviews, 47, 65–81.
[11] C. Sanchez-Moreno, J.A. Larrauri and F. Saura-Calixto, A procedure to measure the antiradical efficiency of polyphenols, J. Sci. Food Agric. 76 (1998), pp. 270–276.
[12] Z. Sroka and W. Cisowski, Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids, Food Chem. Toxicol. 41 (2003), pp. 753–758.
[13] E. Psomiadou and M. Tsimidou, Stability of virgin olive oil. 1. Autoxidation studies, J. Agric. Food Chem. 50 (2002), pp. 716–721.
[14]WHO, Geneva (Sept. 1997). Manual on antimicrobial resistance and susceptibility testing. Diversion of emerging and other communicable diseases surveillance and control. WHO antimicrobial resistance monitoringprogramme.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


