Lateral Line System in Fish: Structure and Function
| ✅ Paper Type: Free Essay | ✅ Subject: Biology |
| ✅ Wordcount: 2785 words | ✅ Published: 12 Jun 2018 |
Introduction
The lateral line is a sensory system in fish and amphibians. It is made up of mechanoreceptors called neuromasts which are sensitive to water movement (Diaz et al. 2003). The lateral line system has an important role in the detection of stationary objects, navigation, prey detection, capture and in swimming in schools (Gelman et al. 2007).
The receptor organ of the lateral line system is the neuromast. There are two types of neuromasts, canal neuromasts which are located in the intradermal canals, and the superficial neuromasts which are located in the intraepidermal canals. Canal neuromasts are able to detect water flow acceleration, while superficial or free neuromasts can detect velocity (Gelman et al. 2007).
In some species like the American paddlefish (Polyodon spathula), the lateral line system has evolved into an electrosensory system (Modrell et al. 2011). This was accomplished by the specialization of hair cell receptors. These hair cell receptors in the lateral line system resemble the sensory hairs of insects. This may suggest that both derive from a common ancestral mechanosensory organ (Dambly-Chaudiere et al. 2003).
This review paper will focus on the lateral line systems anatomy, function and its components. It will also consider the origin of the lateral line system, modifications of the lateral line and explore research gaps in the literature.
Origin of the Lateral Line System
A study undertaken by Robert H. Denison explained the origin of the lateral line system. The author explained that early vertebrates had a pore-canal system in the dermis which functioned as a primitive sensory system detecting water movement. Through embryology and comparative anatomy, it has been established that the inner ear is closely related to the lateral line system (Denison 1966).
The inner ear and the lateral line are developed from ectodermal thickenings, called dorso-lateral placodes. These have a number of similarities, including receptors with sensory hairs, and are both innervated by fibers in the acoustico-lateral area of the brain (Denison 1966).
Early vertebrate fossils revealed that the pore canal system which consists of canals that lie below the dermis, and pore canals which connect the canals that lie below the dermis to the surface. The pore canal system is present and developed in Osteostraci which is a group of ostracoderms. It is present in Heterostraci which is another group of ostracoderms and includes early vertebrates such as lungfishes and crossopterygians. As its presence is extensive, it is reasonable to suggest that the pore canal system was a primitive character in early vertebrates (Denison 1966).
The author states that this relationship between the pore canal system and the lateral line was first recognized in Osteotraci. In transverse sections, canals that are located below the dermis in the pore canal system are difficult to be distinguished from a lateral line canal (Figure 1). Both of these canals have a narrow opening and a basal part which is separated by a horizontal septum into an outer part that is filled with mucus, and an inner part which consists of sensory cells and nerves (Denison 1966).
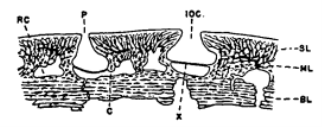
Figure 1. The figure shows a transverse section of an ostcostracan. This depicts the similar structure of the lateral line canal (IOC) and a canal of the pore canal system (P). BL represents the basal layer, C is the canal which connects the mesh canal with the vascular canal. ML represents the middle layer, RC the vascular canal, SL the superficial layer and X represents the septum that separates the lateral line canal (Denison 1966).
As the structure between these two systems is similar the author determined that the lateral line was derived from pore a canal system, and then became a specialized part of it and later remained there (Denison 1966).
Structure of the Lateral Line System
Organization of the Lateral Line
The lateral line, consists of a row of small pores which lead into the underlying lateral line canal. In the head, the lateral line canal is separated into three canals, one passes forward and above the eye, another forward and below the eye and the other downward and below the jaw (Figure 2) (Parker 1904). These three canals have numerous pores and together with the lateral line canal, make the lateral line system.
Epidermal structures called neuromasts form the peripheral area of the lateral line. Neuromasts consist of two types of cells, hair cells and supporting cells. Hair cells have an epidermal origin and each hair cell has one high kynocyle (5-10 μm) and 30 to 150 short stereocilia (2-3 μm). The number of hair cells in each neuromast depends on its size, and they can range from dozens to thousands. Hair cells can be oriented in two opposite directions with each hair cell surrounded by supporting cells. At the basal part of each hair cell, there are synaptic contacts with afferent and efferent nerve fibers. Afferent fibers, transmit signals to the neural centres of the lateral line and expand at the neuromast base. The regulation of hair cells is achieved by the action of efferent fibers (Jakubowski 1967).
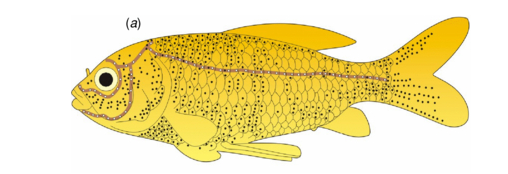
Figure 2. Diagram of the lateral line system. The lateral line canal is divided into 3 stems, one passes forward and above the eye, another forward and below the eye and the other downward and below the jaw. Black dots represent the location of the neuromasts on the skin surface. White dots on the brown line show the positions of the neuromasts in sub-epidermal lateral line canals (Yang et al. 2010).
Stereocilia and kinocilium of hair cells are immersed into a cupula and are located above the surface of the sensory epithelium. The cupula is created by a gel-like media, which is secreted by non-receptor cells of the neuromast (Figure 3). There are two types of neuromasts, superficial or free neuromasts and canal neuromasts. Superficial neuromasts are located at the surface of the body and are affected by the environment. Superficial neuromasts are categorized into primary or paedomorphic neuromasts and secondary or neomorphic neuromasts. Canal neuromasts are primary neuromasts. These are found inside epidermal or bony canals and are located on the head or body of the fish (Coombs et al. 1992).
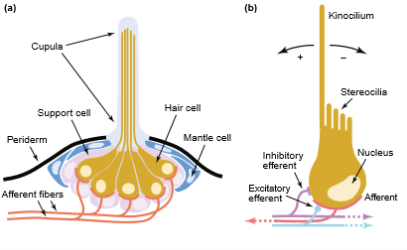
Figure 3. Lateral line of fish. (a) The figure shows the basic structure of neuromasts and all its components. (b) Hair cell, depicting the innervation of afferent and efferent fibers (Dambly-Chaudiere et al. 2003).
Superficial and Canal Neuromasts
Superficial neuromasts are small and can be found in lampreys, teleost fishes and in some bony fishes. Superficial neuromasts are located on the head and the body and in some fish in the caudal fin (Cernuda et al. 1996).
They have a cylindrical cupula and a round base with a diameter that can seldom reach 100 μkm. The number of hair cells is small, from several dozens to several hundred (Cernuda et al. 1996).
In canal neuromasts, the sensory area is situated at the bottom of the canal below the skin. Canal neuromasts have a large range in size, shape and orientation within the canal. Some species have narrow canals and the neuromast can be found in a local constriction with the long axis running parallel to the canal axis. Some other fishes have neuromasts which are found in wide canals and have a different shape. Canal neuromasts allow the efficient detection of pressure differentials, which are created by the current movement across the canal pores (Cernuda et al. 1996).
Lateral Line System Function
The lateral line system has often been described as ”touch at a distance”. This is due to the lateral line function being similar to the senses of touch and hearing (Coombs et al. 2006). The earliest hypothesis about the function of the lateral line was that it secretes mucus to cover the body. Several years later, it was determined that the lateral line is used to detect water current and stimuli from moving objects (Bleckmann et al. 1993).
Fish can sense water movements ranging from large-scale currents to small disturbances caused by plankton. This is due to the superficial neuromasts which are able to respond to very weak water currents, with speeds from 0.03 mm/s and higher. Canal neuromasts can respond to current speeds from 0.3 to 20 mm/s (Bleckmann et al. 1993). The lateral line has functions in schooling, prey detection, spawning, rheotaxis (which is a form of taxis when fish face an ongoing current), courtship and station holding (Coombs et al. 2006).
It is thought that the lateral line system can create hydrodynamic images of the surrounding area. This can be achieved by detecting moving and stationary objects in active and passive ways. Active hydrodynamic imaging is similar to the echolocation of objects that is observed in dolphins. Here, fish produce a flow field around their body, which helps them in detecting distortions in their flow field. This is observed in blind cavefishes, which rely on this mechanism to explore their surroundings. For example, they are able to differentiate between structures that differ by even 1 mm (Coombs et al. 2006).
Passive hydrodynamic imaging can be carried out for moving and stationary bodies. This is achieved by detecting currents that are generated by other moving bodies such as other fish or the movement of stationary objects such as rocks in a stream (Coombs et al. 2006).
Lateral Line Information Processing
Lateral line information is processed in all regions of the brain (Figure 4). The information is provided by afferent nerve fibres and is sent to the brain via the lateral line nerves that enter the ipsilateral brainstem and terminate in the medial octavolateralis nucleus (MON). Main primary lateral line projections reach the ipsilateral cerebellar granular eminence while the second order of projections from the medial octavolateralis nucleus terminate in the lateral compartment of the torus semicircularis and in the deep layers of the optic tectum. The final pathway for information processing is the relay of information from the midbrain to different diencephalic nuclei (Bleckmann 2008).
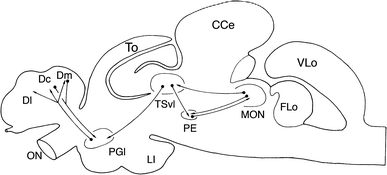
Figure 4. This figure depicts the pathway of information processing. MON represents the medial octavolateralis nucleus, CCe represents the Corpus cerebelli, Ll is the hypothalamic inferior lobe, Flo is the facial lobe, ON is the olfactory lobe, PGl represents the lateral preglomerular nucleus, PE is the pre eminential nucleus, TSvl is the Ventro lateral nucleus of torus semicircularis (Bleckmann 2008).
Lateral Line Modifications
The lateral line system of elasmobranchs is different to that of teleost fish. Elasmobranchs have superficial neuromasts and two morphological classes of sub-epidermal canals. Elasmobranch canals have skin pores that allow direct contact with the surrounding water. They may also have absent skin pores which prevent the contact of canal fluid with the external environment. In teleost fish, hydrodynamic pressure differences at the skin pores cause fluid motion. This results in pored canal neuromasts being able to cipher the acceleration of external water flow near the skin, and induce behaviours such as hydrodynamic imaging, detection of prey and schooling. In elasmobranch fishes, other than prey detection the function of the lateral line pores and their neurophysical response is not yet known (Maruska and Tricas 2004).
Sharks and batoids have non-pored canals which are located on the ventral body surface, rostrum and around the mouth (Figure 5). The absence of skin pores demonstrates that localized weak hydrodynamic flow which causes pressure differences will not produce canal fluid motion directly, as it occurs in the pored canal systems (Maruska and Tricas 2004).
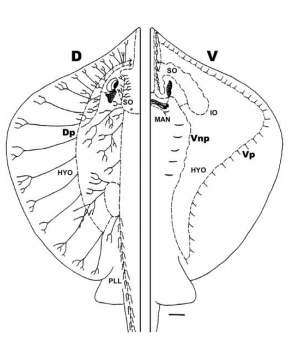
Figure 5. Lateral line canal system on the dorsal (D) and ventral (V) surface of the Atlantic stingray, Dasyatis Sabina. Solid lines indicate neuromast-free tubules which terminate in pores. The other lines indicate canal sections which consist of innervated neuromasts (Maruska and Tricas 2004).
A hypothesis was developed to explain the function of non-pored canals in elasmobranch fishes. The hypothesis explains that the non-pored canals of stingrays which are located on the ventral surface, function as tactile receptors that aids in the localization and capture of small benthic prey. The hypothesis explains that direct coupling of the skin and canal fluid should result in an increase in sensitivity to the velocity of skin movement, which would mean that primary afferents that innervate neuromasts would show characteristics consistent with detectors of velocity. The hypothesis also states that without direction to the external environment, non-pored canals will have lower sensitivity to water motion in comparison to tactile stimulation (Maruska and Tricas 2004).
A study done by Karen P. Maruska and Timothy C. Tricas (2004) determined that pored hyomandibular canals on the stingrays’ dorsal surface are different in terms of primary afferent response from the non-pored hyomandibular canals on the ventral surface. They expressed that primary afferents from the dorsal pored canals respond as hydrodynamic acceleration detectors of water disturbances which are mainly caused by predators. Ventral non-pored canals are sensitive to small movements of the skin, and primary afferents encode the velocity of fluid induced in the canal by these stimuli. The results supported their main hypothesis and demonstrate the function of the lateral line in elasmobranchs in prey detection (Maruska and Tricas 2004).
Research Gaps
At present, we have a good understanding of how the brain stem and the midbrain respond to different types of stimuli for example, a change in water flow or movement of an object. However, we know nothing about information processing in the tectum opticum which forms the roof of the midbrain and functions as the primary visual center. In amphibians the tectum opticum, a lateral line map is created which helps in registering with a visual and an electrosensory map, which together represent the external area (Parker 1904).
Furthermore, we have no information on how lateral line information is processed in cerebellum, which is a brain structure that is involved in motor control and also has a role in cognition. Additionally, little is known about the process of adaptation in the lateral line pathway and how the efferent pathway in the electrosensory lateral line functions in gaining control which is thought to apply in the mechanosensory line (Parker 1904).
There is not a lot of information on the internal and chemical structure of the cupula, and how the cupula is attached to the base of the neuromast. The role of the lateral line in schooling is poorly understood. In elasmobranch fishes, other than prey detection the function of the lateral line pores and their neurophysical response has not been fully researched.
Conclusion
The lateral line system which is a sensory system in fish and amphibians has various functions in schooling, navigation, and prey detection. Through paleontology, comparative anatomy and embryology it was demonstrated that there is a phylogenetic connection between the pore canal system in the dermis of early vertebrates and the lateral line. Moreover, through the action of neuromasts and hydrodynamic imaging, the fish is able to detect its surrounding environment. Lastly, there are some research gaps regarding on how lateral line information is processed in certain parts of the brain.
Literature Cited:
Bleckmann, H. and Zelick, R. (1993) The Responses of Peripheral and Central Mechanosensory Lateral Line Units of Weakly Electric Fish to Moving-Objects. Journal of Comparative Physiology A-Sensory Neural and Behavioral Physiology, 172 (1), pp. 115-128.
Bleckmann, H. (2008) Peripheral and central processing of lateral line information. Journal of Comparative Physiology A-Neuroethology Sensory Neural and Behavioral Physiology, 194 (2), pp. 145-158.
Cernuda Cernuda, R. and Garcia Fernandez, J. (1996) Structural diversity of the ordinary and specialized lateral line organs. Microscopy Research and Technique, 34 (4), pp. 302-312
Coombs, S., Jansenn, J. and Montgomery, J. (1992) Functional and Evolutionary Implications of Peripheral Diversity in Lateral Line Systems.
Coombs, S. and van Netten, S. (2006) The Hydrodynamics and Structural Mechanics of the Lateral Line System. Fish Biomechanics, 23, pp. 103-139.
Dambly-Chaudiere, C., Sapede, D., Soubiran, F., Decorde, K., Gompel, N. and Ghysen, A. (2003) The lateral line of zebrafish: a model system for the analysis of morphogenesis and neural development in vertebrates. Biology of the Cell, 95 (9), pp. 579-587.
Denison, R. (1966) Origin of Lateral-Line Sensory System. American Zoologist, 6 (3), pp. 369-371.
Diaz, J., Prie-Granie, M., Kentouri, M., Varsamos, S. and Connes, R. (2003) Development of the lateral line system in the sea bass. Journal of Fish Biology, 62 (1), pp. 24-40.
Gelman, S., Ayali, A., Tytell, E.D. and Cohen, A.H. (2007) Larval lampreys possess a functional lateral line system. Journal of Comparative Physiology A-Neuroethology Sensory Neural and Behavioral Physiology, 193 (2), pp. 271-277.
JAKUBOWSKI, M. (1967) Cutaneous Sense Organs of Fishes .7. Structure of System of Lateral-Line Canal Organs in Percidae. Acta Biologica Cracoviensia Series Zoologia, 10 (1), pp. 69-81.
Maruska, K. and Tricas, T. (2004) Test of the mechanotactile hypothesis: neuromast morphology and response dynamics of mechanosensory lateral line primary afferents in the stingray. Journal of Experimental Biology, 207 (20), pp. 3463-3476.
Modrell, M.S., Bemis, W.E., Northcutt, R.G., Davis, M.C. and Baker, C.V.H. (2011) Electrosensory ampullary organs are derived from lateral line placodes in bony fishes. Nature Communications, 2, pp. 496.
Parker G.H (1904) Bulletin of the Bureau of Fisheries. 24th edition. Washington Government Printing Office, pp 180-204
Yang, Y., Nguyen, N., Chen, N., Lockwood, M., Tucker, C., Hu, H., Bleckmann, H., Liu, C. and Jones, D.L. (2010) Artificial lateral line with biomimetic neuromasts to emulate fish sensing. Bioinspiration & Biomimetics, 5 (1), pp. 016001.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


