Evolution of Respiratory Systems in Animals
| ✅ Paper Type: Free Essay | ✅ Subject: Biology |
| ✅ Wordcount: 4508 words | ✅ Published: 12 Jun 2018 |
- Jonathan Codd
Respiration in animals is a necessity as it allows the exchange of respiratory gases that are required for survival. There are huge variations in the designs of respiratory systems and each has evolved due to selective pressures in environments, such as food and territory. The evolution of species is driven in part by limited resources and the adaptations in which these can be exploited. This report will describe the evolution from aquatic environments to terrestrial environments as part of this movement, of animals onto land, created a cause for the fast development of newly designed systems in order to support air breathing as oppose to gaining oxygen from water using gills. Lungfish first developed lungs, and the ability to breathe air instead of water, whilst living in aquatic environments and the appearance of air-breathing in fish is the major foundation for terrestrialization. Each species has different requirements due to different techniques of movement and feeding, for example, and respiratory systems are required to support the lifestyle of each species in order to exchange the maximum amount of gas possible. The ability to develop additional processes able to assist in respiration has ensured that the oxygen demands of each animal is met.


The respiratory system of animals is crucial for the life as it allows the exchange of gases between an organism and the environment. These respiratory systems have been forced to continually develop new designs depending on new evolutionary pressures from changing environments. Many species have evolved due to the availability of new niches and unexploited resources and thus have been forced to develop supporting mechanisms of respiration. This report examines the evolution of respiration from aquatic environments to the terrestrialization of land and the rapid expansion of respiratory methods that soon followed. The evolution of lungs from gills in the Sarcopterygii lineage has allowed the tetrapod transition onto land and is responsible for the ability to eventually develop fully terrestrial species that are able to respire solely air. Each system must be complementary to the requirements of the species and environment in order to meet the aerobic demands and some species are able to undergo various methods of respiration in order to undergo sufficient rates of gaseous exchange. Each method has been specifically developed for the niche, and uncinate processes have formed in order to assist with ensuring respiration can be as efficient as possible.
Introduction
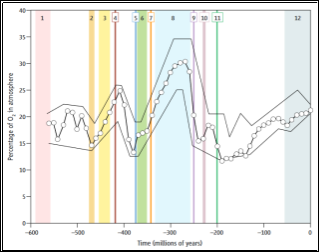
The evolution of air breathing was vital in the transition of life from aquatic to terrestrial environments and, therefore the rapid evolution of the animal kingdom’s physiology and anatomy in order to exploit all available niches (Graham, 1997). The development of air breathing would not, however, have taken place if the atmospheric composition had not altered during the Phanerozoic era, around 550 million years ago, when the concentration of oxygen showed an increase, likely due to the appearance of large vascular land plants (Ra et al., 2007). The ozone layer was thought to have formed around two billion years ago (Walker, 1978) and is essential for allowing the survival of life on earth by preventing high-energy ultra-violet radiation from entering the earth’s atmosphere (Parson, 2003). The movement onto land has allowed for a huge expansion in the amount of available niches and therefore caused a rapid radiation in the body plans of animals and a variety of respiratory mechanisms to evolve in support (Ra et al., 2007)
The Importance of Pulmonary Surfactant
Lungs differ throughout the animal kingdom as they have evolved for the specific niche of each species; nevertheless one thing they all commonly include is a gas-liquid interface which allows surface tension to arise, causing complications (Daniels and Orgeig, 2003). The pulmonary surfactant system prevents the collapse of respiratory surfaces in lungs due to unequal pressures arising from differently sized alveoli, as well as maintaining a reduced resistance to air flow and improving lung compliance (Daniels and Orgeig, 2003). There is overwhelming evidence that there was a single evolutionary origin of the surfactant system, thought to be from the epithelial cells lining the pharynx (Daniels et al., 2004), due to Surfactant Protein-A (SP-A) or like-structures being present in all the major vertebrate groups; implying that it is an essential pre-requisite for lung evolution (Sullivan et al., 1998). Surfactant has been studied in swim bladders, which have now been shown to be a homolog of the lung, with the original principal function being an anti-adhesive but also with involvement in preventing water from entering the swim bladders or lungs (Daniels et al., 2004)
Pulmonary surfactant composition is primarily lipids (around 90%) most of which are phospholipids, and the remaining ten percent is comprised of proteins. (Veldhuizen et al., 1998). There were found to be four types of surfactant proteins (SPs): A, B, C and D which all have varying properties and roles within the surfactant system; SP-B and SP-C were both found to be highly involved in the surface activity due to hydrophobic properties and SP-D is hydrophilic and part of the collectin family (Wüstneck et al., 2005). Dipalmitoylphosphatidylcholine (DPPC) is the most hydrophobic lipid component and therefore DPPC-rich monolayers are able to sit packed tightly together, ensuring the exclusion of water, however they are not well suited for the expansion of the lungs and so are alternated with mixed monolayers when necessary (Wüstneck et al., 2005).
Respiration in Fish
Fish evolution has allowed both water and air breathing to arise as a means of gas exchange and as these vary greatly in properties, such as density and the oxygen concentration, the mechanistic pumps must also show great diversity to meet the requirements for effective respiration (Brainerd and Ferry-Graham, 2005).
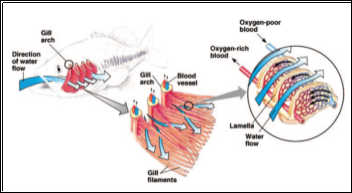

Fish that breathe in water use gills which are highly evolved organs that provide large surface areas and thin barriers between the fish’s blood and the aquatic environment, thus allowing for a high rate of gaseous diffusion (Evans et al., 2005). Whilst they show properties for gaseous exchange the gills are multifunctional organs which are also responsible for the loss of ions and nitrogenous waste, therefore fish must also have regulatory mechanisms allowing them to successfully osmoregulate (Evans et al., 2005). As fish are continually moving they all require a buccal pressure pump as well as a suction pump, most likely the operculum, working in tandem to allow for expansion and compression to move water across the gills; the suction pump is more prominent in some species such as the Osteichthyes compared to the Chondrichthyes (Ap and La, 2001). A counter-current method is established due to water flowing in the opposite direction to the movement of blood, with the secondary lamellae being the site of gaseous exchange (Shelton and Randall, 1962). The counter-current mechanism is required as the content of dissolved oxygen is less in water than it would be in the atmosphere (Ibanez et al., 2008) and thus allows high concentrations of gas to be exchanged, whereas a con-current mechanism would too quickly reach an equilibrium and efficient extraction of oxygen would cease (Brainerd and Ferry-Graham, 2005).
There are two hypothesis surrounding the origin of air breathing in fish, one suggests that lungs arose only once at the base of the Osteichthyes, whereas opposing arguments have recently suggested that lungs evolved on at least two separate occasions and instead developed in both the Actinopterygii and Sarcopterygii (Brainerd, 1994). Some air breathing fish, such as the Actinopterygian, are able to modify their buccal pump to create a four-pump mechanism, using two expiration and compression cycles, in which expired air is first pumped into the lungs before being compressed out into the atmosphere (Perry et al., 2001).
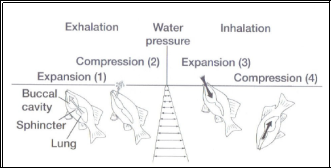

When empty fresh air is inhaled via the expansion of the buccal cavity before finally being compressed into the lungs, this is shown to fully expand and compress and so there is little mixing of expired and fresh air (Perry et al., 2001). Not all air breathing fish show this mechanism as some Dipnoi, lungfish for example, will still ventilate using the primitive two-stroke mechanism (Burggren and Johansen, 1986) and even though there can be mixing of expired and fresh air in the lungs, there has been no significant evidence to suggest that this is any less effective than the four-stroke method as breathing accessories allows the increase in volume of inhaled gases (Brainerd, 1994).
Terrestrialisation
Lungs were an obvious pre-requisite for the transition onto land but there were many other anatomical and physiological adaptations necessary for tetrapods before they were able to fully terrestrialise and survive free of an aquatic environment (Daeschler et al., 2006). In the late Devonian, terrestralisation occurred as a means of exploring previously unexploited niches and resources through the evolution of tetrapods via the Sarcopterygian lineage, whose habitats were most likely mud-flats neighbouring the waters edge (Graham and Lee, 2004).

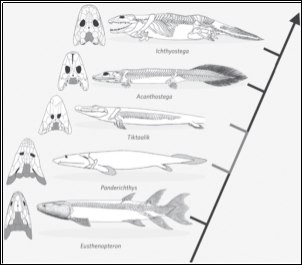
Tetrapods share common features with both modern day land vertebrates and fishes and the discovery of Tiktaalik rosaea allowed the transitional form to be studied in detail to provide evidence on the necessary adaptations required for terrestrialisation (Sarfati, 2007). The skeleton of Tiktaalik was found to be a lot stronger than that of its sarcopterygii-like ancestors and would have allowed it to support its own body weight in substrate, it also showed a longer snout and loss of bony gill covering but still maintained fish-like qualities, such as well developed gill arches and fin rays which implies that it still spent a majority of time in an aquatic environment (Ahlberg and Clack, 2006). Tetrapod digits were seen to arise from the pectoral fins of Sarcopterygii, although there was seen to be a pattern there remained a few anomalies throughout the development (Sarfati, 2007).
When discovered Tiktaalik was a hugely important addition to the fossil record and bridged the gap between fish and tetrapods after confirmation from phylogenetic studies placed it on the Sarcopterygian to tetrapod lineage (Sarfati, 2007). The further anatomical and physiological changes that continued after Tiktaalik were responsible for allowing tetrapods to adopt new mechanisms of feeding and locomotion that were required for survival on land and thus was responsible for a huge step in the necessary radiation of respiratory systems (Clack, 2006).
Respiration in Amphibians and Non-Avian Reptiles
Amphibians are able to breathe by utilising cutaneous methods, using their skin to exchange gases, which could also suggest that it was an important method of respiration used during the transition onto land (Gans, 1970). [JC1]Some amphibians, that have a large enough surface area to volume ratio, such as certain species of salamanders, will rely solely on cutaneous respiration for gaseous exchange due to an absence of lungs (Feder and Burggren, 1985). Cutaneous respiration is based on an infinite pool of oxygen, through air or water mediums, in what is known as a co-current or open flow and is a passive process as there is a lack of inspiratory or expiratory flow (Burggren and Moallf, 1984). Whilst a few amphibians will rely only on cutaneous respiration, most will just use it as an accessory breathing mechanisms and will have other primary methods of respiration (Brainerd and Owerkowicz, 2006).
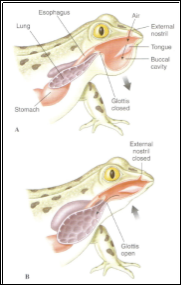

As a means of understanding the primitive breathing in early tetrapods and air breathing fish, other species of salamanders have been studied. It was found that they use a method similar to the two-stroke mechanism previously described in Dipnoi; in which during inspiration they will expand their buccal cavity in order to create a negative pressure required to draw in fresh air, therefore supporting claims that this is most likely the primite mechanism of respiration seen in Sarcopterygii and early tetrapods (Brainerd et al., 1993).
Whilst most air breathers will create a negative pressure to cause air to move into the lungs, frogs and some air breathing fish, are known as positive pressure breathers as they use their buccal chamber to fill with air which they will then actively force into the lungs (Jones, 1982). In frogs this system consists of two valves; the paired nares, which remain open for the majority of the time with the function of connecting the buccal cavity to the external air and the glottal valve which spends the majority of the time closed and is only opened when air is entering or leaving the lungs from the buccal chamber (Jones, 1982). This breathing cycle is most likely to begin with expiration as breath-holding was found to most likely occur during the end of the buccal inspiration (Jones, 1982).
Reptiles, are believed to be the first group of animals to involve movements of the ribs in the assisting with lung ventilation (Nielsen, 1961). Aspiration breathing is thought to have arison in amniotes, which includes reptiles and mammals, most of which have tried to eliminate their reliance on costal aspiration by evolving accessory breathing methods to aid in respiration (Brainerd and Owerkowicz, 2006). It is evident that aspiration breathing evolved after the buccal pump mechanisms, however, there have been no transitional forms intermediate between the two found which suggests that aspiration breathing developed abruptly and amniotes soon after lost the ability to utilise a buccal cavity (Brainerd, 1999). Studies in lepidosaurs, established that most have an unidivided pleural cavity, which is also present in amphibians and air breathing fish, suggesting that this is the primitive form and that seperation occurred only later on in evolutionary history (Brainerd, 1999).
Crocodiles display many unique features compared to the rest of the reptile group as they use a hepatic pistol to ventilate their lungs by utlising a muscle known as the diaphragmaticus, which is not homologous with any other diaphragmatic muscle (Brainerd, 1999). The liver divides the thoracoabdominal cavity and the diaphragmaticus muscle, orinating from the pelvis and caudal gastralia, is responsible for the expansion of the thoracic cavity by retracting the liver; this creates a negative pressure inside and fresh air is forced in, with inspiration containing an intermediate pause (Brainerd and Owerkowicz, 2006). The multicameral chamber seen in crocdiles allows high aerobic demands to be met, which is vital for their survival, and is only found in few other reptile species (Perry, 1988).
Respiration in Avian Reptiles
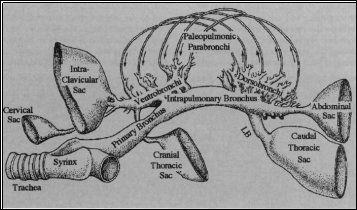
Avian reptiles, more commonly known as birds, use a lung-air sac respiratory system which allows cross-current flow, where air and blood are flowing in the same direction to one another (Scheid and Piiper, 1972). The avian respiratory system is small and compact and the thin barriers are thought to be advantageous during flight but not necessary as the mammalian bat respiratory system is dissimilar but still successful for long migratory flights (Schmidt-Nielsen, 1997). Uncinate processes, which alternate depending on the niche of each bird, are fundamental in the avian respiratory system and assist with the movement of the ribs and sternum, allowing for both inspiration and expiration to take place (Codd et al., 2008).
The air sacs are used only for ventilation, with gaseous exchanges taking place as air is passed through the parabronchi, which are thin tubes with openings at each end allowing the uni-directional flow of air, which was found to be unique to avian respiration (Scheid, 1979). The parabronchi are packed into a dense hexagonal array with gas exchange tissue, known as the mantle, surrounding the lumen of each; composing a networks of both blood and air capillaries (Brown et al., 1997). The cross-current system found in birds requires these blood and air capillaries to be in close proximity and arranged parallel to one another in order for diffusion to take place; with the uni-directional flow being studied and found to be of no additional advantage to this cross-current system (Scheid, 1979).
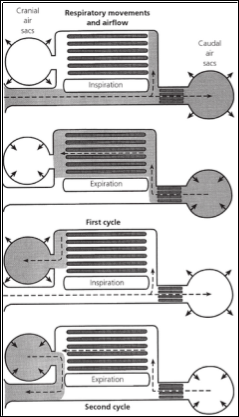

There are a total of two inspiratory and expiratory cycles that must occur for the complete flow of air through the lungs (Schmidt-Nielsen, 1997). During the first inspiration air flow is split from the trachea to the caudally grouped air sacs or the dorsobronchi, where it will enter the parabronchi and the gas that remained in the lungs from the previous inspiration is forced cranially (Brown et al., 1997). When the first expiration takes place the air remaining in the caudal air sacs moves through the parabronchi, where gas exchange takes place, and another inspiration forces the air into the cranial air sacs (Brown et al., 1997). To exit the respiratory system, the second expiration forces the air to flow from the cranial air sacs through the ventrobronchi and exits using the trachea (Reece et al., 2015).
The trachea involved in avian respiration is made up of complete cartilagenous rings and is found to be around 4.5 times the size of mammalian homologues which allows larger tidal volumes and increased compliance within the system (Reece et al., 2015). There have been no valves discovered in the avian respiratory system and therefore to maintain unidirectional air flow it has been suggested that aerodynamics methods, such as jet flow, must be existing in the system during inspiration, and increased resistance through the intrapulmonary bronchus is used during expiratory flow (Scheid, 1979).
Respiration in Mammals
The respiratory system in mammals is completely separated from the abdominal cavity and the diaphragm and ribs are both crucial in the mechanism of respiration (Keith, 1905). The muscles of the ribs, such as the intercostals, are required for the expansion of the ribcage, which allows the neccesary generating of negative pressure caused by increased the lung volume for aspiration breathing (Perry et al., 2010).
The diaphragm is responsible for the control of inspiration as it is able to contract and elongate the thoracic cavity which creates a negative pressure, thus drawing air into the lungs (Loh et al., 1977). The pericardium is closely bound to the lungs and is connected to the central tendon of the diaphragm allowing the vital pairing of both (Keith, 1905). The elevation of the rib cage, which allows further increase in the available volume for external air to enter the lungs, is also under diaphragmattic control (Loh et al., 1977).
The mammalian lung is highly complex and involves lots of branching in order to increase surface area from the trachea, which then splits in series into the primary bronchi, secondary bronchi, tertiary bronchi and finally the alveoli. There are around 3×108 alveolar air sacs which comprise of thin membranes to increase the surface area and allow the ease of diffusion of gases between them and the capillaries (Hoppensteadt and Peskin, 2002). Inspiration and expiration cycling allows the constant renewal of air into and out of the lungs and provides mammalian species with sufficient oxygen to meet the aerobic demands (Weibel, 1984).
Respiration in Insects

There is a wide variety of mechanisms adopted by insects for respiration due to the huge variation in available niches. All will utilise a network of air-filled vessels, which are known as tracheae and tracheoles, and can be as small as 1μm in diameter, with most terminating nearby to the mitochondria of cells (Miller, 1966). The tracheal system at rest is filled with fluid which is thought to be actively absorbed by the permeable inner tracheal wall when required for breathing, using active transport or secretion from cells (Wigglesworth, 1953). Experiments have demonstrated that during tracheal compression, which is controlled by an increased pressure inside the exoskeleton, the tracheae noticeably shrink in diameter to aid in air convection and increased diffusion of oxygen into the tissues due to a high pressure build up (Westneat et al., 2003).
Spiracles are required at the external and internal barrier in the tracheael system to allow external air through the skin; and previous experiments have found if these are blocked then the insect cannot survive as respiration will cease (Fraenkel and Herford, 1938). Interneurons are essential as they are responsible for the pairing of spiracle movement with ventilation by communicating with the spiracle’s motor neurones (Miller, 1966). The discontinuous gas exchange cycles present in insects will typically occur in three stages, beginning the closed-spiracle phase where only small amounts of external gas exchange are able to take place (Lighton, 1996). A fluttering-spiracle phase permits oxygen uptake for the diffusion of gases into the tracheael tissues and finally an open-spiracle phase concludes the cycle whilst allowing the release of accumulated carbon dioxide (Lighton, 1996).
Ventilation is under endogenously controlled rhythms produced by the central nervous system which allows aerobic respiration rates in flight muscles to be so successful that they can be challenged only by certain species of bacteria (Miller, 1966). During insect respiration air is sucked into the tracheal system by creating negative alterations in internal pressure using the pumping of hemolymph by the heart or the contracting of abdominal muscles, others can include passive diffusion or autoventilation (Westneat et al., 2003).
Respiration in Cetaceans
Cetaceans have evolved a much more unusual respiratory system to any terrestrial mammal, as the nasal passageway has moved to a more dorsal position to allow the exclusion of water from the system and ease of breathing as they surface (Thomas and Kastelein, 1991). A nasal plug, made up of nasal plug muscle, connective tissue and adipose tissue, is responsible for the seperation of the internal and external environment and is retracted anteriolaterally for respiration by bilaterally paired nasal plug muscles (Thomas and Kastelein, 1991).
The lung size of cetaceans varies depending on the depth of the dives undertaken, due to the variety of pressures causing differing extents of thoracic collapse (Piscitelli et al., 2010). It was found that the lung size will be reduced in cetaceans that undergo deeper dives and there will be an increase in the thoracic mobility. The lungs of larger whales were found to possess extremely heavy myoelastic bundles in the air sacs and alvolar membranes that were not found in much smaller cetacea (Wislocki, 1942).
Conclusion
The evolution of respiratory systems has been an extremely specific process that has showed both gradual, and rapid changes throughout the many lineages of the animal kingdom in order to encorporate universal requirements, as well as accessory breathing mechanisms (Weibel, 1984). Respiration is a vital life process required for survival and it is essential that gas exchange is as efficient as possible in order to allow high oxygen-demanding aerobic activities to take place when necessary (Perry, 1988).
Each respiratory system may have a variety of additional mechanisms, uncinate processes, that assist in the breathing mechanics to enable the ease of transporting larger volumes of air within each system (Codd et al., 2008). The pulmonary surfactant system is of great importance, as it allows the successful existance of such mechanisms by preventing collapse of respiratory surfaces, as well as aiding them by increasing lung compliance and reducing the resistance to air flow (Daniels and Orgeig, 2003).
The evolution of efficient respiratory systems, when paired with other necessary adaptations, has provided a foundation for more complex body systems to develop to allow the utilisation of previously unexploited resources and niches, thus providing organisms advantages in the animal kingdom (Graham, 1997).
Bibliography
Ahlberg, P.E., Clack, J.A., 2006. Palaeontology: A firm step from water to land. Nature 440, 747-749. doi:10.1038/440747a
Ap, S., La, F.-G., 2001. Ventilatory modes and mechanics of the hedgehog skate (Leucoraja erinacea): testing the continuous flow model. J. Exp. Biol. 204, 1577-1587.
Brainerd, E.L., 1999. New perspectives on the evolution of lung ventilation mechanisms in vertebrates. Exp. Biol. Online 4, 1-28. doi:10.1007/s00898-999-0002-1
Brainerd, E.L., 1994. The Evolution of Lung-Gill Bimodal Breathing and the Homology of Vertebrate Respiratory Pumps. Integr. Comp. Biol. 34, 289-299. doi:10.1093/icb/34.2.289
Brainerd, E.L., Ditelberg, J.S., Bramble, D.M., 1993. Lung ventilation in salamanders and the evolution of vertebrate air-breathing mechanisms. Biol. J. Linn. Soc. 49, 163-183. doi:10.1111/j.1095-8312.1993.tb00896.x
Brainerd, E.L., Ferry-Graham, L.A., 2005. Mechanics of Respiratory Pumps, in: Physiology, B.-F. (Ed.), Fish Biomechanics. Academic Press, pp. 1-28. doi:10.1016/S1546-5098(05)23001-7
Brainerd, E.L., Owerkowicz, T., 2006. Functional morphology and evolution of aspiration breathing in tetrapods. Respir. Physiol. Neurobiol., Frontiers in Comparative Physiology II: Respiratory Rhythm, Pattern and Responses to Environmental Change 154, 73-88. doi:10.1016/j.resp.2006.06.003
Brown, R.E., Brain, J.D., Wang, N., 1997. The avian respiratory system: a unique model for studies of respiratory toxicosis and for monitoring air quality. Environ. Health Perspect. 105, 188-200.
Burggren, W., Moallf, R., 1984. “Active” regulation of cutaneous exchange by capillary recruitment in amphibians: Experimental evidence and a revised model for skin respiration. Respir. Physiol. 55, 379-392. doi:10.1016/0034-5687(84)90059-8
Burggren, W.W., Johansen, K., 1986. Circulation and respiration in lungfishes (dipnoi). J. Morphol. 190, 217-236. doi:10.1002/jmor.1051900415
Clack, J.A., 2006. The emergence of early tetrapods. Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 167-189. doi:10.1016/j.palaeo.2005.07.019
Codd, J.R., Manning, P.L., Norell, M.A., Perry, S.F., 2008. Avian-like breathing mechanics in maniraptoran dinosaurs. Proc. R. Soc. Lond. B Biol. Sci. 275, 157-161. doi:10.1098/rspb.2007.1233
Daeschler, E.B., Shubin, N.H., Jenkins, F.A., 2006. A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature 440, 757-763. doi:10.1038/nature04639
Daniels, C.B., Orgeig, S., 2003. Pulmonary Surfactant: The Key to the Evolution of Air Breathing. News Phsiology Sci. 18, 151-157.
Daniels, C.B., Orgeig, S., Sullivan, L.C., Ling, N., Bennett, M.B., Schürch, S., Val, A.L., Brauner, C.J., 2004. The Origin and Evolution of the Surfactant System in Fish: Insights into the Evolution of Lungs and Swim Bladders. Physiol. Biochem. Zool. Ecol. Evol. Approaches 77, 732-749. doi:10.1086/422058
Evans, D.H., Piermarini, P.M., Choe, K.P., 2005. The Multifunctional Fish Gill: Dominant Site of Gas Exchange, Osmoregulation, Acid-Base Regulation, and Excretion of Nitrogenous Waste. Physiol. Rev. 85, 97-177. doi:10.1152/physrev.00050.2003
Feder, M.E., Burggren, W.W., 1985. Cutaneous Gas Exchange in Vertebrates: Design, Patterns, Control and Implications. Biol. Rev. 60, 1-45. doi:10.1111/j.1469-185X.1985.tb00416.x
Fraenkel, G., Herford, G.V.B., 1938. The Respiration of Insects Through the Skin. J. Exp. Biol. 15, 266-280.
Gans, C., 1970. Respiration in Early Tetrapods-The Frog is a Red Herring. Evolution 24, 723-734. doi:10.2307/2406552
Graham, J.B., 1997. Air-Breathing Fishes: Evolution, Diversity, and Adaptation. Academic Press.
Graham, J.B., Lee, H.J., 2004. Breathing Air in Air: In What Ways Might Extant Amphibious Fish Biology Relate to Prevailing Concepts about Early Tetrapods, the Evolution of Vertebrate Air Breathing, and the Vertebrate Land Transition? Physiol. Biochem. Zool. 77, 720-731. doi:10.1086/425184
Hoppensteadt, F.C., Peskin, C.S., 2002. Gas Exchange in the Lungs, in: Modeling and Simulation in Medicine and the Life Sciences, Texts in Applied Mathematics. Springer New York, pp. 75-108. doi:10.1007/978-0-387-21571-6_3
Ibanez, J.G., Hernandez-Esparza, M., Doria-Serrano, C., Fregoso-Infante, A., Singh, M.M., 2008. Dissolved Oxygen in Water, in: Environmental Chemistry. Springer New York, pp. 16-27. doi:10.1007/978-0-387-49493-7_2
Jones, R.M., 1982. How toads breathe: Control of air flow to and from the lungs by the nares in Bufo marinus. Respir. Physiol. 49, 251-265. doi:10.1016/0034-5687(82)90077-9
Keith, A., 1905. The Nature of the Mammalian Diaphragm and Pleural Cavities. J. Anat. Physiol. 39, 243-284.
Lighton, J.R., 1996. Discontinuous gas exchange in insects. Annu. Rev. Entomol. 41, 309-324. doi:10.1146/annurev.en.41.010196.001521
Loh, L., Goldman, M., Davis, J.N., 1977. The assessment of diaphragm function. Medicine (Baltimore) 56, 165-169.
Miller, P.L., 1966. The Regulation of Breathing in Insects, in: J.W.L. Beament, J.E.T. and V.B.W. (Ed.), Advances in Insect Physiology. Academic Press, pp. 279-354. doi:10.1016/S0065-2806(08)60189-7
Nielsen, B., 1961. On the Regulation of the Respiration in Reptiles. J. Exp. Biol. 38, 301-314.
Parson, E.A., 2003. Protecting the Ozone Layer: Science and Strategy. Oxford University Press.
Perry, S.F., 1988. Functional Morphology of the Lungs of the Nile Crocodile, Crocodylus Niloticus: Non-Respiratory Parameters. J. Exp. Biol. 134, 99-117.
Perry, S.F., Similowski, T., Klein, W., Codd, J.R., 2010. The evolutionary origin of the mammalian diaphragm. Respir. Physiol. Neurobiol. 171, 1-16. doi:10.1016/j.resp.2010.01.004
Perry, S.F., Wilson, R.J.A., Straus, C., Harris, M.B., Remmers, J.E., 2001. Which came first, the lung or the breath? Comp
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


