Case Study: Methanol Poisoning of a Child
| ✅ Paper Type: Free Essay | ✅ Subject: Sciences |
| ✅ Wordcount: 2575 words | ✅ Published: 16 Apr 2018 |


Introduction
In this PBL, we observe a case of methanol poisoning in a child. We will first define the unfamiliar term of tertiary care centre and proceed to understand acid base homeostasis in the body, the basis for methanol poisoning. Then we will look at how ethanol and methanol are metabolised in the body followed by how methanol poisoning actually works. We will also discuss how the osmolal gap is obtained using osmolality and osmolarity and lastly child abuse.

Learning Objectives
- Definition of unfamiliar terminology
- An overview of acid base homeostasis in the body
- How ethanol and methanol are metabolised in the body
- How does methanol poisoning work
- Osmolality and osmolarity
- Child abuse

1. Unfamiliar term: Tertiary care centre
A tertiary care centre is where a patient goes to when primary and secondary care have not been able to adequately treat the patient. Tertiary care centres are equipped with highly trained staff and highly dedicated medical equipment to cater to complex treatments or procedures as required by the patient. An example of a tertiary care centre would be the colorectal unit at The Royal London (1). Amareen was transferred to a tertiary care centre to receive more suitable care mainly due to the fact that she was so young and suffering from methanol poisoning.
2. Overview of acid base homeostasis in the body
Acid base homeostasis is the regulation of hydrogen ions. The higher the concentration of hydrogen ions, the lower the pH and vice versa. Acidic solutions have a high pH whereas alkaline solutions have a lower pH. The normal pH in the body is in the range of 7.35-7.45. A pH lower than 7.35 results in acidosis whereas a pH higher than 7.45 results in alkalosis.
Acid base balance has its basis in the Henderson-Hasselbalch equation shown in Figure 2. If we rearrange the equation, we see that bicarbonate and carbon dioxide directly affects the acid base balance.

Figure 2: Henderson-Hasselbalch Equation (3)
There are three main ways in which the body controls the acid base balance. These three systems usually work together. Firstly, there are physiologic buffers, each of which consist of a weak acid and its base salt or a weak base and its base acid. Physiologic buffers react immediately within seconds to the change in pH in the body. These buffer systems occur in both intra and extracellular parts of the cells. The main buffering systems for physiologic buffers are extracellular bicarbonate-carbonic acid buffering system, intracellular protein buffers and phosphate buffers in the bone. An overview of the physiologic buffer system is shown below in Figure 1.

Figure 1: Physiologic buffer systems (2)
If physiologic buffers are not enough to return the pH back to its normal value, pulmonary compensation can take place in the lungs. This works by eliminating or retaining carbon dioxide. Increased ventilation effort (hyperventilation) and decreased ventilation effort (hypoventilation) are the two ways pulmonary compensation works. The changes in pulmonary compensation is rapid within a few minutes.
The final compensatory mechanism, renal compensation in the kidney, starts when the previous two mechanisms have failed to regulate the pH level in the body. The kidneys maintain balance by excreting or conserving bicarbonate and hydrogen ions in the body. However, this compensatory mechanism is a long term regulator and takes longer, usually a few hours, to respond to a change in acid base balance.
The normal arterial blood gas values for partial pressure of carbon dioxide is 35-45 mmHg/ 4.7 kPa-6.0 kPa and bicarbonate concentration is 22-26 mmol/L. A change in the partial pressure of carbon dioxide or bicarbonate concentration from normal levels results in respiratory or metabolic disorders respectively. This together with acidosis or alkalosis determined by the pH results in 4 main disorders arising. Figure 3 below shows the 4 different disorders and their respective compensatory mechanisms.

Figure 3: Diagnosis of the four main acid base disorders and respective compensation mechanisms (4).
In Amareen’s case, metabolic acidosis occurs. The major cause is the high production of formic acid which is not excreted quickly from the body. This has caused a decrease in the bicarbonate concentration due to H+ combining with bicarbonate. By the law of mass action using the Henderson- Hasselbalch equation, there is extra CO2 generated thus Amareen shows respiratory compensation via tachypneic breathing whereby the peripheral chemo receptors in the lungs are stimulated which stimulates the alveoli to try to exhale the excess CO2. The exhalation of CO2 would in most cases be enough to correct the metabolic acidosis but in this case as methanol was ingested, external treatment was mandatory to save Amareen’s life.
3. How methanol and ethanol are metabolised in the body
Ethanol metabolism
Ethanol, commonly known as drinking alcohol, is metabolised primarily by alcohol dehydrogenase in the liver. Once ingested, ethanol is quickly absorbed by the gastrointestinal tract and small intestines with concentrations reaching at maximum level at 20-60 minutes (5). Ethanol is metabolised to acetaldehyde by alcohol dehydrogenase and then to acetate in the mitochondria via aldehyde dehydrogenase. Acetate is then metabolised to Acetyl CoA and subsequently to CO2 and H2O by the Krebs cycle in the mitochondria. Ethanol can also be metabolised by two other pathways: by cytochrome P450 2E1 (CYP2E1) in microsomes of the endoplasmic reticulum when there is a high ethanol consumption and by catalase in peroxisomes (6). The three ways ethanol is metabolised are illustrated in Figure 4 below.
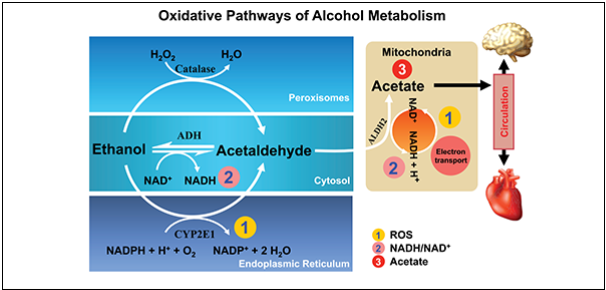
Figure 4: Oxidative pathways of ethanol metabolism in the body (7)
Methanol metabolism
Methanol, commonly known as wood alcohol, can be absorbed by the body via inhalation, ingestion and skin exposure (8). In this specific case, Amareen ingests methanol. When methanol is ingested, it is quickly absorbed by the gastrointestinal tract with concentration reaching a maximum level at 30-90 minutes after ingestion (8). Methanol is primarily metabolised in the liver. In the first step, methanol is metabolised to formaldehyde by alcohol dehydrogenase. Then formaldehyde is subsequently metabolised to formic acid by formaldehyde dehydrogenase. Formic acid is finally metabolised into carbon dioxide and water in the presence of tetrahydrofolate. This final step proceeds very slowly and hence there is an accumulation of formic acid in the body which is the chief cause of methanol poisoning and will be discussed in the next section. Methanol metabolism is illustrated in Figure 5 below.

Figure 5: Metabolism of methanol (9)
4. How does methanol poisoning work
Symptoms and Causes
The main characteristics of methanol poisoning are metabolic acidosis and ocular damage. Formic acid, the metabolite of methanol and not methanol itself is considered to be toxic. The severity of the toxicity correlates with the degree of metabolic acidosis rather than concentration of methanol. (10, 11) The accumulation of formic acid in the body has many detrimental effects if left untreated. The effects of methanol poisoning can be grouped into different phases (12). The phases are described in Table 1 below.
|
Phase |
Comments |
|
1 |
Phase 1 is also called the latent phase. Slight central nervous system depression occurs which is usually followed by an asymptomatic latent period which can last 8-24 hours after ingestion of methanol. This is the case with Amareen who appeared normal when she first arrived at the hospital and was examined |
|
2 |
Phase 2 involves the development of metabolic acidosis due to the build-up of formic acid. This can cause abdominal pain, confusion, vomiting and blurred vision some of which were seen in Amareen upon arriving at the tertiary care centre. |
|
3 |
Phase 3 is characterised by haemorrhage in the basal ganglia of the brain and major ocular damage which can lead to blindness. This is believed to be due to the inhibition of retinal hexokinase by formaldehyde (13). The patient may also exhibit hypotension, coma and Kussmaul breathing. |
Table 1: Different phases of methanol poisoning
Formic acid has been shown to inhibit cytochrome C oxidase activity in mitochondria (14) which is similar to the action of cyanide, hydrogen sulphate and carbon monoxide (15). Cytochrome C oxidase is the last enzyme in the electron transport chain of the mitochondria which results in the synthesis of ATP (16). Thus, by inhibiting cytochrome C oxidase, there would be significant reduction in the synthesis of ATP resulting in cell hypoxia leading to cell injury and death (17, 18).
The amount of formic acid in the blood is proportional to the increase in the anion gap which measures the contribution of unmeasured anions to acidosis by using the formula [Na+] + [K+] – [Cl-] – [HCO3-] (19). A high anion gap of 20mmol/dL was observed in the arterial blood gas of Amareen when she was transferred to the tertiary care centre. A high anion gap indicates the loss of bicarbonate ions without concurrent loss in chloride ions. Thus, a low serum bicarbonate level is a reliable indicator of the severity of methanol poisoning. Other causes of a high anion gap are diabetes keto acidosis, lactic acidosis, ethylene glycol and salycilate.
Diagnosis
Diagnosis for Amareen was relatively easy as a history was available from her parents stating that she had ingested methanol. This allowed doctors to treat Amareen quickly and correctly to prevent blindness or even death. If a history is unavailable, a test for the osmolal gap (refer to on how osmolal gap is derived) is very useful. A high osmolal gap (>10 mOsm/kg H20) indicates the presence of significant amounts of low molecular weight substances such as methanol. When methanol is metabolised, the osmolal gap returns to the normal and the anion gap increases due to formic acid formation which causes bicarbonate ions to decrease via the Henderson Hasselbach equation.
A high serum methanol concentration of 35 mg/dL (> 0mg/dL), low serum bicarbonate level of 18mmol/L, low pH of 7.32 and a high anion gap of 20mmol/dL confirms Amareen’s diagnosis of metabolic acidosis cause by methanol poisoning.
Treatments
In this case, Amareen is treated with an ethanol drip. This is because like methanol, ethanol uses alcohol dehydrogenase as its first stage of metabolism and that ethanol has a higher affinity for alcohol dehydrogenase than methanol in the ratio of 20:1 (8). Therefore when ethanol enters the bloodstream, they will competitively bind to alcohol dehydrogenase thus inhibiting the formation of formic acid. In a clinical setting, a target level of 100-150 mg/dl is used to saturate alcohol dehydrogenase with ethanol (20). However, ethanol can be a challenge to administer due to irregular rate of metabolism making a steady target level difficult to maintain and it can also cause intoxication (20).
Amareen later receives fomepizole treatment after the ethanol drip was not so effective. This is a better treatment because fomepizole has an even higher affinity for alcohol dehydrogenase than methanol in the ratio of 8000:1 (21). This prevents methanol from being metabolised. The advantages of fomepizole are the ease of administration, long duration of effect and that it doesn’t cause intoxication (22). However, fomepizole is very expensive and is less widely available (23).
Due to both ethanol and fomepizole being ineffective in reducing the serum concentration of methanol in the body, haemodialysis was started. Haemodialysis is the most effective way to remove methanol and formic acid from the body (24). This works by passing blood from the body through a dialysis machine that contains a series of membranes to filter out unwanted substances and replenish essential minerals to the blood and then pumping blood back to into the body. The reason why haemodialysis was not immediately administered was probably due to it requiring a neck line which is very invasive and can result in multiple complications for Amareen who is only 5 years old.
5. Osmolarity and Osmolality
Osmolality refers to the osmolar concentration of plasma per kilogram of solvent. Osmolality is measured using osmometers. Osmolarity on the other hand refers to the osmolar concentration of plasma per litre of solution. This value is calculated using a set formula from measured concentrations of Na+, K+, glucose and urea. The equation is 2[Na+] + 2[K+] + Glucose + Urea (all in mmol/L). Using osmolality and osmolarity, the osmolal gap can be calculated which is the difference between the actual osmolality and the calculated osmolarity which normally lies in the range of 8-10 mOsm/kg (25).
6. Child Abuse
There are four main categories of child abuse (26). Physical abuse which involves bodily harm for example bruises, burns and fractures. Emotional abuse that involves persistent emotional ill-treatment or neglect causing adverse effects on the child’s emotional development. Sexual abuse by forcing a child to perform sexual activity. This includes ‘non-contact’ sexual activities such as producing child pornography. Lastly, negligence which is the failure of carers to provide the basic physical and psychological needs as well as supervision from harm to the child which results in an adverse effect on the child’s health and development. An example would be protecting a child from dangerous substances which Amareen’s parents have failed to do.
References
- NHS. Barts Health – General surgery for patients: NHS; 2014 [cited 2014 11 November]. Available from: http://www.bartshealth.nhs.uk/our-services/services-a-z/g/general-surgery/for-patients/.
- College AC. Electrolyte Fluid Balance: Austin Community College; 2014 [cited 2014 11 November]. Available from: http://www.austincc.edu/apreview/EmphasisItems/Electrolytefluidbalance.html.
- Keener P. Okeanos Explorer | Expeditions | INDEX 2010: Indonesia-USA Deep-Sea Exploration | Expedition Purpose 2014 [cited 2014 11 November]. Available from: http://oceanexplorer.noaa.gov/okeanos/explorations/10index/background/edu/purpose.html.
- Droual R. The Urinary System: Fluid and Electrolyte Balance: Modesto Junior College; 2014 [cited 2014 11 November]. Available from: http://droualb.faculty.mjc.edu/Course%20Materials/Physiology%20101/Chapter%20Notes/Fall%202011/chapter_19%20Fall%202011.htm.
- Jones AW, Jonsson KA, Neri A. Peak blood-ethanol concentration and the time of its occurrence after rapid drinking on an empty stomach. J Forensic Sci. 1991;36(2):376-85.
- Zimatkin SM, Deitrich RA. Ethanol metabolism in the brain.: Addiction Biology; 1997. p. 387-400.
- Zakhari S. Alcohol metabolism and epigenetics changes. Alcohol Res. 2013;35(1):6-16.
- (IPCS) IPoCS. Methanol. Environmental Health Criteria 196. Geneva: WHO; 1997.
- Stürmann K, Ryan MT. Alcohol-Related Emergencies:A New Look At An Old ProblemEmergency Medicine Practice. 2001;3(9):9.
- Jacobsen D, McMartin KE. Antidotes for methanol and ethylene glycol poisoning. J Toxicol Clin Toxicol. 1997;35(2):127-43.
- Swartz RD, Millman RP, Billi JE, Bondar NP, Migdal SD, Simonian SK, et al. Epidemic methanol poisoning: clinical and biochemical analysis of a recent episode. Medicine (Baltimore). 1981;60(5):373-82.
- Tephly TR. The toxicity of methanol. Life Sci. 1991;48(11):1031-41.
- Martin-Amat G, McMartin KE, Hayreh SS, Hayreh MS, Tephly TR. Methanol poisoning: ocular toxicity produced by formate. Toxicol Appl Pharmacol. 1978;45(1):201-8.
- Nicholls P. The effect of formate on cytochrome aa3 and on electron transport in the intact respiratory chain. Biochim Biophys Acta. 1976;430(1):13-29.
- Alonso JR, Cardellach F, Lopez S, Casademont J, Miro O. Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol Toxicol. 2003;93(3):142-6.
- Fontanesi F, Soto IC, Barrientos A. Cytochrome c oxidase biogenesis: new levels of regulation. IUBMB Life. 2008;60(9):557-68.
- Shah S, Pandey V, Thakore N, Mehta I. Study of 63 cases of methyl alcohol poisoning (hooch tragedy in Ahmedabad). J Assoc Physicians India. 2012;60:34-6.
- Jammalamadaka D, Raissi S. Ethylene glycol, methanol and isopropyl alcohol intoxication. Am J Med Sci. 2010;339(3):276-81.
- Sejersted OM, Jacobsen D, Ovrebo S, Jansen H. Formate concentrations in plasma from patients poisoned with methanol. Acta Med Scand. 1983;213(2):105-10.
- Jacobsen D, McMartin KE. Methanol and ethylene glycol poisonings. Mechanism of toxicity, clinical course, diagnosis and treatment. Med Toxicol. 1986;1(5):309-34.
- Bestic M, Blackford M, Reed M. Fomepizole: a critical assessment of current dosing recommendations. J Clin Pharmacol. 2009;49(2):130-7.
- Hall TL. Fomepizole in the treatment of ethylene glycol poisoning. Cjem. 2002;4(3):199-204.
- Rathi M, Sakhuja V, Jha V. Visual blurring and metabolic acidosis after ingestion of bootlegged alcohol. Hemodial Int. 2006;10(1):8-14.
- Suki WN, Massry SG. Therapy of renal diseases and related disorders: Springer; 1991.
- Kapur G, Valentini RP, Imam AA, Jain A, Mattoo TK. Serum osmolal gap in patients with idiopathic nephrotic syndrome and severe edema. Pediatrics. 2007;119(6):e1404-7.
- NICE. When to suspect child maltreatment. July 2009.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


