Neutralisation of False Positives using Presumptive Tests
| ✅ Paper Type: Free Essay | ✅ Subject: Sciences |
| ✅ Wordcount: 3431 words | ✅ Published: 15 Sep 2017 |
Jasnique Tiwana
The Neutralisation of False Positives using Presumptive Tests for Blood
Introduction
This project emphasis upon neutralising presumptive tests for blood, this differs from other studies as they just test for false positives, instead of attempting to neutralise these known false positives. This project was conducted as there are no current obvious projects on the neutralisation of false positives. This is a crucial topic because it will save time at crime scenes when looking at a suspected blood stain, many false positives are known to interfere with the presumptive tests kits. However, if these can be eliminated at the scene, then it will save lots of time conducting confirmatory tests for a stain that is not blood. It will be interesting to discover whether one neutralizer can neutralise all or the majority of the unknown false positives.
The aims of this project are to find out which substance/ products produce false positives, and whether or not these false positives can be neutralised. In addition, how many of these false positives work for both Kastle-Meyers and Leucomalachite green. Also whether the type of surface these false positives are found upon affects the ability for them to be detected.
There are numerous amounts of different bodily fluids that can be found at a crime scene. Blood is one of the most common and important bodily fluid found at a crime scene as it can give an insight to a DNA profile and much more. The blood can either be from the offender this can help corroborate stories and give a deeper insight into what actually happened (The Forensics Library, n.d). In the criminal justice system blood is defined as “a vital complex biological fluid containing red blood cells, which is present in vertebrate and may be shed during an accidental, intentional and/or criminal acts.” (Wonder,2001). Blood consist of erythrocytes, leucocytes and platelets, hence presumptive assays test for the presence of haemoglobin located in the erythrocytes (Jackson and Jackson, 2007).
However, at a scene it may not always be obvious as to whether or not the stain is actually blood, thus, presumptive tests are required to determine whether the stain could be blood or not. There are various different presumptive tests designed to identify whether the stain is blood, although, these are not a confirmatory test for blood. Blood found at a crime scene is essential as it can provide a DNA profile from both the victim and the suspect. (Gupta, et, al. 2016). This can then be collected for further analysis in the laboratory, to obtain DNA profiles etc. (Tobe, et, al. 2007). Over centuries various different attempted clean-up methods have been used for blood, including bleach, therefore, it is important to use a presumptive test which can detect microscopic blood stains.
Presumptive tests
Presumptive tests with the exception of luminol are not applied directly to a stain, instead, the suspected stain is lifted using a sterile swab or filter paper, the presumptive test is then carried out on the filter paper or the swab. This is to ensure that the suspected stain is not damaged. In certain cases, such as where the surfaces have been washed down, it becomes more appropriate to use luminol due to its high level of detection (Jackson and Jackson, 2007).
Presumptive tests are used by forensic scientists worldwide to aid in the identification of unknown substances such as blood. However, a presumptive test does not provide definitive identification instead it provides useful information which helps to decide what further action if any is needed.
There are numerous presumptive tests for blood, historically with the most common being benzidine which was first introduced in 1904 (after Kastle-Meyers) which was introduced in 1901). Moreover, this is no longer widely used in forensics as it was discovered to have carcinogenic effects. Tetramethylbenzidine has also been recognised as a carcinogen and caution should be applied when using either test (James,1998).
Presumptive tests are not specific to Human blood as they will also give a positive result for animal blood. Commonly, a colour change is observed to determine whether it is positive for that substance, due to these presumptive tests are subject to false positives and false negatives. In screening tests for blood, the heam group is observed as this acts as a catalyst which is involved in the chemical reactions.
False positive results can be obtained from chemicals containing strong oxidants such as bleaches and household cleaners. Plant peroxidases such as horseradish can also affect presumptive tests such as horseradish as they contain peroxidases they catalyse oxidation reactions thus causing a false positive. They are known to be sensitive to heat so heating up a plant peroxidase can inactivate it. A false positive is identified as a colour change before the addition of hydrogen peroxide (Li,2008). Li (2008) states that although uncommon false negatives can also occur, this happens when a strong reductant is present, this hinders the oxidation reaction.
Luminol
Luminol is referred to a chemiluminescent reaction which is oxidised by haemoglobin. It was a very early method used in 1937 to detect microscopic amounts of blood at a crime scene as it has a very small detection limit (in nanograms). Although, the test is sensitive it is also prone to false positives as with the other presumptive tests of blood. Sodium Hypochlorite is in bleach based substances commonly used for a clean-up, this would be detected by the luminol test (Quickenden and Cooper 2001). A limitation to luminol is that it must be used in total darkness to be able to see the reaction. It is different from the other presumptive tests as it involves illumination with a bright light (blue) (Webb, et al, 2006).
Kastle-Meyers
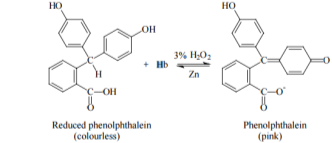
The phenolphthalin Assay is referred to as the Kastle Meyers test, it differs from phenolphthalein which is a class of dye indicator commonly used in titrations. Phenolphthalin is a colourless compound (the reduced form), the oxidized derivative is phenolphthalein which turns pink (Li, 2015). The Kastle-Meyers (KM) test is one of the most popular presumptive test used by forensic scientists, it is possible to detect blood up to 100,000x dilution (Bell, 2012). Figure 1 shows the oxidation of phenolphthalein causing a pink colour change when reacting with a peroxide.
The Kastle-Meyers test is a presumptive test used to identify blood stains; it contains phenolphthalein, which reacts with the haemoglobin in blood with the addition of hydrogen peroxide leading to a pink colour change for a positive test. This test, however, is not specific for blood and can be subject to false positives, it is also not specific to human blood and will react with animal blood. Therefore, it is not a confirmatory test of blood. Leucomalachite green (LMG) is also a presumptive test of blood, however; it is not as popular as the Kastle Meyers test (Bell, 2012). A positive result for Leucomalachite green is a green-blue colour change.
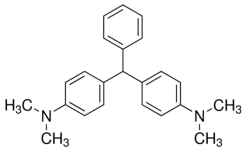
Figure 1 to show the chemical structure of Leucomalachite green (Sigma Aldridge, 2017)
Leucomalachite Green chemical symbol C23H26N2 (PubChem, 2005), this is also a colour change test it is not as widely used as Kastle – Meyers but it is just as useful. Leucomalachite green is oxidised by haem causing a blue-green colour change it is colourless when in its reduced state. The reaction is usually carried out in acetic acid and hydrogen peroxide which acts as an oxidiser (li, 2015). It is used in a very similar way to Kastle-Meyers, as the test is almost identical, this test as with the Kastle- Meyers, therefore, it is also prone to a false positive, hence further analytical tests are required to confirm if its blood. Due to the false positives, it cannot be called a confirmatory test for blood. These tests are also not specific as they do not react to just human blood but other animal blood.
Neutralising agents
Various different neutralisers are available for bleach. Bleach is known to be a common false positive detected with Presumptive tests. The active ingredient in Bleach is Sodium Hypochlorite, therefore, research into is neutralisation was looked into. The most common one used is Sodium Thiosulphate another is Ascorbic acid which is used more commonly in water storage tanks to climate all remaining bleach making the water safe to drink (Tanguay,2013). Due to the neutralisation of peroxide both these neutralisers will be used to see if they neutralise all the false positives.
Method
Preparation of the reagents:
The Kastle-Meyers test: The reagent is made by weighing out 12g of phenolphthalein, 120g of potassium hydroxide and 30grams of Zinc dust and 600ml of distilled water should then be added. This is then put onto a hot plate and stirred for 3 hours. (Langford et,al).
The Kastle-Meyers was used as follows, the stain was moistened with ethanol (optional depending on how the stain was lifted) and this is rubbed over the stain, then two drops of the Kastle-Meyers is then added onto the stain, at this stage a colour change is indicative of a false positive, if there is no false positive at this point then continue to the next stage, and add two drops of 3% hydrogen peroxide, if there is an immediate pink colour change this indicates blood could be present as it is a positive result for blood. If there is no colour change then it can be almost certainly said that no blood is present, this is due to its high level of sensitivity.
Leucomalachite green solution was made up using 0.11grams of Leucomalachite green powder, 66mL of glacial acetic acid and 33mL of distilled water, this was mixed together to form the colourless Leucomalachite green (cox, 1991). The same method mentioned above is used for Leucomalachite green with a blue-green colour change being a positive result for blood and a blue-green before the addition of hydrogen peroxide being a false positive. The initial Leucomalachite green solution is clear.
Defibrinated horse blood was used due to its similarity to human blood, also because presumptive tests cannot differentiate between blood types. The swabs used were all in sterile packaging and all the pipettes had been autoclaved prior to use to ensure that all equipment used was sterile as not to allow any cross contamination. Blood was placed straight onto a sterile swab; this was subjected to the presumptive tests to ensure no other contaminants caused a colour change this acted as a positive control. Negative controls were created using a sterile swab and adding each of the reagents to make sure nothing else was interfering with the test. A positive control was also taken by swabbing blood from denim to see if it would still react or if there was an interfering factor.
To determine the levels of sensitivity for both KM and LMG blood was diluted and put into test tubes. Blood was pipetted into test tubes using Finn pipettes in order to determine accuracy. Different concentrations of blood were made up using distilled water and blood. The solutions made up were 1:10, 1:100, 1:1000, 1: 10,000, 1: 100,000 were prepared. This was done by pipetting 1mL of blood into 9mL of water, from this solution 1mL was added into 9mL of water and so on. This method was used to determine the level of sensitivity of the presumptive tests used a sterile swab was dipped into each of these solutions and the Kastle -Meyers test was performed. The time taken for these to turn and the shade of pink in which they turned were recorded. Each swab was taken 3 times in order to determine accuracy, but also each test tube was made up 3 times, this gave a total of 15 test tubes, to make sure no extra or no less blood was added each time, and to check the reliability of the method as the blood had begun slightly clotting. This was done over a series of days to see if the results differed in anyway.
A series of household items and known false positives obtained from previous literature reviews were determined and these were placed onto the denim material, by rubbing the item onto the material. The items used were, horseradish, bleach, red onion, lemon, tomato, tomato ketchup (Heinz) and potato. These were then allowed to dry on the material before dampening the stain with distilled water. This was repeated three times and also repeated after 3 days when it had more time to dry.
With a fresh batch of false positives, horseradish, bleach, ketchup and tomato were re tested to see if they reacted to the new Kastle Meyers and Leucomalachite green solutions.
Once the false positives were established such as the use of bleach, then sodium thiosulphate and Ascorbic acid were added to the stains to see if they affected the tests and gave a new negative reaction.
Sodium thiosulphate was made up using 0.25g of Sodium Thiosulphate and 5ml of water and mixed to make a 5% solution, the crystals were stirred and the remaining solution was colourless
Ascorbic acid was made up using 0.25g of ascorbic acid in 10ml of water, this was then stirred and gives a clear solution.
Firstly, a sterile was dipped into bleach and a few drops of the leucomalachite green were applied using a disposable sterile pipette each time, the colour change was then observed. Once this was established another swab was dipped into bleach but this time sodium thiosulphate was first added to the swab, (again using a disposable sterile swab) the sodium thiosulphate was applied all the way around the swab to ensure all areas were covered. In addition, lecucomalachite green was then pipetted onto the swab and observed for a colour change, the same thing was then repeated using ascorbic acid. The swab was held up against a white background to see if It had a slight reaction or not. It was tried with pure bleach and diluted bleach to see if the reactions were the same. The neutralising agents were also used with pure blood to see if it reacted the same with blood and caused a colour change, hydrogen peroxide was added to the pure blood stain. This was to determine whether or not it is a true false positive or not.
The next stage was to repeat the following steps using Kastle -Meyers on pure bleach and diluted bleach.
In addition, to determine whether or not the false positives worked on all peroxides, both Ascorbic acid and Sodium Thiosulphate were also used on horseradish, in an attempt to neutralise it, this was repeated three times.
Results
Firstly, a positive and negative control were taken to ensure a positive result was obtained for pure blood and a negative result for water, indicating there was no contamination.
A serial dilution was made for the Kastle-Meyers to determine the level of sensitivity. It was measured to a 1 in 100,000 dilution as this is the results obtained from previous literature as to the level of sensitivity. Table 1 shows the intensity of the colour change from the dilution of blood, this was repeated three times.
Table 1 a table to show the level of sensitivity of the Kastle-Meyers solution
|
Dilution factor |
Repeat 1 |
Repeat 2 |
Repeat 3 |
|
X10 |
Bright pink |
Bright Pink |
Bright Pink |
|
X100 |
Positive |
Positive |
Positive |
|
X1,000 |
Positive |
Positive |
Positive |
|
X10,000 |
Faint pink |
Faint pink |
None |
|
X100,000 |
None |
None |
None |
False positives were first tested on denim to see if they reacted. The blood sample was placed onto the denim material first this was to determine whether the Kastle-Meyers kit was working correctly. The results of the false positives can be seen in table 2, each sample was firstly loaded onto the swab and the denim material, this was too see if there was a difference between the two methods. The highlighted results in table two show the false positives which differed on the denim and the swab. Table 2 shows the first experiment to test for false positives comparing it to the reaction it had on denim.
Table 2 false positives using Kastle-Meyers on denim and directly to swab, with* meaning inconclusive result as it is the same colour in which the test kit changes, the samples reacted after 5 mins of the addition of Kastle-Meyers reagent
|
False positive |
Negative/positive reaction on denim material |
Added directly to swab |
|
Horse Radish root |
– |
– |
|
Bleach(Sodium Hypochlorite) |
– |
+ |
|
Red onion |
– |
– |
|
Potato |
– |
– |
|
Tomato sauce |
– |
+ |
|
Red Onion |
– |
– |
|
Lemon |
– |
+ |
|
Tomato |
– |
* |
|
Red radish |
– |
– |
Leucomalachite green was also tested for its sensitivity of blood
This was then tested on pure blood with the addition of hydrogen peroxide. This was to check if the solution made up gives a blue-green colour change. Table 3 shown below shows a serial dilution for Leucomalachite green, the serial dilution was performed in the same way as in the Kastle-Meyers test and the colour change and intensity of the change was recorded
Table 3 a table to show the dilution factor of Leucomalachite green after the addition of hydrogen peroxide
|
Dilution factor |
Repeat 1 |
Repeat 2 |
Repeat3 |
|
X10 |
Strong Turquoise |
Strong Turquoise |
Strong Turquoise |
|
X100 |
Blue – green |
Light Blue-green |
Blue-green |
|
X1,000 |
Light Blue-green |
Faint Blue-green |
Light Blue-green |
|
X10,000 |
No reaction |
No reaction |
No reaction |
|
X100,000 |
No reaction |
No reaction |
No reaction |
Table 4 shows the false positives on the denim material and direct application to the swab, the first repeat the same method applied as that in Leucomalachite green
Table 4 false positives using denim material and direct application to the swab, this shows the first attempt using Leucomalachite green
|
False positive |
Negative/positive reaction on denim material |
Added directly to swab |
|
Horse Radish root |
– |
+ |
|
Bleach(Sodium Hypochlorite) |
– |
+ |
|
Red onion |
– |
– |
|
Potato |
– |
– |
|
Tomato sauce |
– |
– |
|
Red Onion |
– |
– |
|
Lemon |
– |
– |
|
Tomato |
– |
– |
|
Red radish |
– |
– |
A fresh set of Known false positives were then used, this was tested with both Kastle Meyers and Leucomalachite green respectively, this is shown in table 5 and 6, each one was tested three times and the reaction and the intensity of the reaction are shown. This time instant colour changes were recorded, using a new Kastle- Meyers test Kit, this was done under a fume hood. This time a diluted bleach sample was used to see if it affected the results of bleach.
Table 5 False positives tested using Kastle -Meyers where + means a positive reaction and – is a negative reaction, instantaneous results
|
False positive |
Repeat 1 |
Repeat 2 |
Repeat 3 |
|
Horseradish |
+ strong pink |
+ pink |
+ strong pink |
|
Tomato |
– |
– |
– |
|
Tomato Ketchup |
– |
– |
– |
|
Bleach |
+ Strong Pink |
+strong Pink |
+ Strong Pink |
|
Diluted bleach |
+ weak pink |
+ pink |
+ weak pink |
Table 6 false positives tested using Leucomalachite green where + indicates a positive reaction and – indicates a negative reaction instantaneous
|
False positive |
Repeat 1 |
Repeat 2 |
Repeat 3 |
|
Horseradish |
+ light green |
+ light green |
+ dark green |
|
Tomato |
– |
– |
– |
|
Tomato Ketchup |
– |
– |
– |
|
Bleach |
+ Strong blue/green |
+strong green |
+ Strong green |
|
Diluted bleach |
+ strong green |
+ strong green |
+ strong green |
Table 7 shows the neutralisation of the false positives identified in table 5 and 6 using ascorbic acid and thiosulphate
Table 7 neutralising agents of the false positives for both Kastle-Meyers and Leucomalachite green. where + indicates a positive reaction (colour change) and – indicates a negative reaction (no colour change)
|
False positive |
Kastle- Meyers |
Leucomalachite green |
||||||||||
|
Sodium thiosulphate |
Ascorbic acid |
Sodium thiosulphate |
Ascorbic acid |
|||||||||
|
Horse radish |
– |
– |
+ |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
|
Bleach |
+ |
– |
– |
+ |
– |
– |
– |
– |
– |
+ |
– |
– |
|
Diluted bleach |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
Discussion
The test was repeated three times to see if the results were the same each time. Kastle -Meyers has a level of sensitivity up to 1 in 100,000. This was found to be the level of sensitivity by other authors. Blood was diluted with water; this was not only to test the level of sensitivity but blood is likely to be cleaned up with water of some kind.
Leucomalachite green is not as widely used as Kastle-Meyers, as it has a lower level of sensitivity
This was diluted in blood to check its level of sensitivity it got a reaction up to 1 in 1,000 rather than 1 in 10,000 as suggested by other literature reports.
Denim material had ketchup, tomato, horseradish and blood added to it, however, it did not yield any results as the false positives did not change colour with the addition to lecuomalachite green. Bleach was tested with lecuomalchite green, this gave an instant colour change, bleach was then diluted in water to see if watered down bleach gave the same effect and this also gave a positive reaction.
Sodium thiosulphate neutralised the effect of bleach as the reaction was barely visible using the Leucomalachite green, however, the ascorbic acid worked but not as well as the thiosulphate. This is because it was hard to tell whether the entire swab had been neutralised as it appeared a very faint green colour around the sides, compared to other swabs which appeared unaffected by the neutralising agent chosen. It was only tested on the bleach and horse radish because they were the only substances which had given a false positive reaction,
None of the substances on denim gave a false positive, this leads to further research into indigo dye as a neutraliser.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


