Methods of Lipid Analysis in Food
| ✅ Paper Type: Free Essay | ✅ Subject: Sciences |
| ✅ Wordcount: 3982 words | ✅ Published: 12 Sep 2017 |
Lipids are defined as the biomolecules whose solubility in water is less than that in non-polar solvents. This definition puts structurally distinct classes of compounds such as fatty acids, terpenes, steroids, prostaglandins and carotenes in the same class (Carey & Giuliano, 2014). Other definition for lipid has also been suggested (Eoin, et al., 2005). They perform a variety of functions in living system including but not limited to structural integrity, energy storage, digestion and communication (Nelson & Cox, 2005).
Although lipids are essential for many body functions, not all lipids are required in same amount. Food and Agriculture Organization (FAO) recommends that total fat of the food contribute to 20-35% of energy for adults. This should include 8% saturated fatty acid(SFA), 11% poly unsaturated fatty acid (PUFA) and less than 1% trans fatty acid (TFA) (FAO, 2010). Chances of insulin resistance is more in overweight person in high SFA diet (Lovejoy, et al., 2002). Similarly, excessive TFA intake also increases the risk of cardiovascular disease (Song, et al., 2015).
Fat content also affects taste. It is proposed that fat has a unique taste Oleogustus that is dependent of chain length and is unique from five conventional tastes: sweetness, sourness, bitterness, saltiness and umami (Running, Craig, & Mattes, 2015). Butter produced from milk with high unsaturated fatty acid (UFA) was found to be more spreadable, softer and less adhesive (Bobe, Hammond, Freeman, Lindberg, & Beitz, 2003).
As the fat content and type effects health as well as taste and texture, it is essential to know the fat content of food. Fat sample is characterized by a variety of criteria and methods. The selection of the criteria and method depends upon the sample type, purpose, accuracy, precision, legal requirement as well as available funds. Hence, despite having limited significance for nutritional purpose, total lipid (TL) measurement is widely carried out as many food labelling regulations require the TL report. Similarly, iodine value can be used for unsaturation study when sophisticated chromatography or spectroscopy methods are unavailable (Greenfield & Southgate, Review of methods of analysis, 2003).
This review intends to compile the available methods of lipid analysis of food products. Focus will be given on the type of sample required, result provided, resolving capacity of each criteria and method. Only brief discussion will be done on the theoretical and experimental process of the method.
Sample preparation is very essential part of analysis of lipid sample. A separate section is dedicated to sample preparation.
In order to analyze the lipid, suitable sample must be prepared. If the sample has lipid inside cells (such as meat) the lipid should be extracted from the cells (Christie & Han, Lipid Analysis, 2012). Samples must undergo some pretreatment before they can be used as test sample. Depending on the nature of sample one or more of the following work is necessary.
2.1. Storage Vessel
Plastic container should be strictly avoided to store lipids. Plasticizers can leach into the sample and contaminate it. Glass vessels or Teflon coated vessels should be used. The atmosphere should be argon or nitrogen to prevent oxidation. (Christie & Han, Lipid Analysis, 2012)
2.2. Protection from Oxidation
Unsaturated fatty acids are prone to oxidation from atmospheric oxygen. Once the oxidation starts, autocatalysis accelerates the process. Different products are formed during oxidation and it may follow various mechanisms. Light, heat, metals, enzyme are known to catalyze the reaction (Angelo, 1996) Oxidation interferes with lipid analysis not only by destroying the unsaturated fats but also by formation of conjugated double bonds which show strong absorption at UV, thus providing false positive results (Christie & Han, Lipid Analysis, 2012)
Elimination of oxygen is the major step in prevention of oxidation. Therefore, all steps should be done in nitrogen atmosphere as far as possible. Equipment should be flushed with nitrogen before experiments. Small amount of antioxidant like 2,6-di-tert-butyl-p-cresol, which do not interfere with analytical process, may be added. Excess of these antioxidant should not be used as they can facilitate oxidation in high concentration. (Christie & Han, Lipid Analysis, 2012)
2.3. Drying of Sample
Lipid sample containing water can make analysis difficult and might be a source of error. Solvents cannot penetrate sample with >8% moisture easily. Hygroscopic solvents like diethyl ether can absorb the moisture, decreasing its extraction efficiency. Low moisture also facilitates grinding and increases the surface area of sample (Shahidi & Wanasindara, 2008). Petroleum spirit, the most widely used solvent requires completely dried sample. (Greenfield & Southgate, Review of methods of analysis, 2003)
Depending on the type of water present (free, adsorbed or water of hydration) different methods may be required for water removal (Bradley, Jr, 2010). Care should be taken during drying as high temperature might lead to decomposition and combination of lipids with other components. These associated lipids cannot be extracted by solvents. Lyophilization (freeze-drying) and vacuum drying methods are preferred drying methods. (Shahidi & Wanasindara, 2008)
2.4. Particle size reduction (Grinding)
Solid food sample might need grinding. Grinding increases the surface area and decreases the length through which solvent need to penetrate the sample (Min & Ellefson, 2010). Care should be taken that the particles are not too fine, too much heat is generated or too much moisture is lost. (IUPAC, 1979)
2.5. Hydrolysis
Lipids in food may be bound ionically or covalently with non-lipid components such as carbohydrate or protein. Solvents are not able to extract them efficiently. Therefore, lipid needs to be hydrolyzed with acid or alkali to turn them into free state. Significant error in lipid extraction is reported when no hydrolysis is carried out. (Min & Ellefson, 2010) Hydrolysis also breaks emulsified fat. (Shahidi & Wanasindara, 2008)
Acid hydrolysis is used for most foods except diary and high sugar content food which require alkaline hydrolysis. Hydrolysis is not preferred when the lipid extract needs to be further analyzed for fatty acid components because they can cause decomposition and oxidation of the sample components. (Greenfield & Southgate, Review of methods of analysis, 2003)
The given sample of food might not be entirely lipid. The amount of lipid in the food sample is called total lipid concentration. It is usually expressed as percentage or per 100gm food (Moreau, 2005). Although total lipid is widely used for food labelling and regulation of food composition, it has limited value as it does not provide the type of molecule in the lipid. (Greenfield & Southgate, Review of methods of analysis, 2003)
There are various methods to determine total lipid in food like solvent extraction, non-solvent extraction, instrumental methods. The selection of methods depends on a number of factor which is discussed below.
3.1. Solvent Extraction
In solvent extraction, the lipid component of the food is extracted by dissolving in suitable organic solvent(s). The solvent selectively dissolves the lipid while leaving the non-lipid portion undissolved. The solvent is then evaporated to leave fat residue. Total lipid is then determined gravimetrically as:
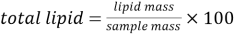 ———————————————————————————-(i)
———————————————————————————-(i)
The above data gives the total fat. However, a significant portion of the fat includes glycerol (from triglycerides) phospholipids and other unsaponifiable matters. Thus, corrections are required so as to represent the correct amount of fatty acids in the sample. The correction factor is provided by FAO. (Greenfield & Southgate, Appendix 5, 2003)
The extracted portion of solvent extraction is highly dependent on solvent use. Hence selection of solvent is discussed in detail next.
|
Ideal solvent should extract all lipids and lipids only. However, due to wide range of polarity of different lipid types, no single solvent can provide an ideal solution. Moreover, the solvent selected should preferably be low boiling, non-flammable, non-toxic in liquid as well as solid, easily disposable after extraction, inexpensive and non- hygroscopic. It should also penetrate sample thoroughly (Min & Ellefson, 2010). Petroleum ether is the most commonly used solvent for its selectivity towards lipid, cost and availability. However, diethyl ether is better solvent for lipids but its fire hazard and hygroscopic nature makes it less favorable than petroleum ether. Ethyl ether and petroleum ether is also sometimes used alternately or together for extraction. (Pomeranz & Meloan, 1994) n-hexane is preferred for oil extraction. (IUPAC, 1979) Mixture of polar and non-polar solvents has shown to extract all the lipids from most food. However, care should be given so as to prevent extraction of unwanted portion. The lipids extracted by this method without hydrolysis is suitable for further treatment to determine fatty acid fractions. (Greenfield & Southgate, Review of methods of analysis, 2003) Alcohol-ether can be used to remove fat from tissue. Water-butanol is used in cereals. Chloroform-methanol is preferred for animal tissue (Pomeranz & Meloan, 1994). |
Solvent extraction is the standard method of analysis for many types of food. Hence, it is widely used and is undergoing continuous improvement. There are different types of solvent extraction, each with its pros and cons.
3.1.1. Batch Extraction
Batch extraction is a very simple, yet widely used method of extraction. The sample is mixed with one or more solvent which along with endogenous water (if any) forms multiple layer of varying concentration. As the lipids are more soluble in non-polar solvents than in water, lipid portion goes to the layer with more solvents and non-lipid component remains in the layer with more water. The lipid part is then separated using a separating funnel. The separation is based on partition principle hence multiple extraction of the aqueous phase is necessary to obtain most of the lipid. The weight of lipid not extracted is given by the equation below: (Pomeranz & Meloan, 1994)
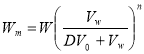 —————————————————————————————————-(ii)
—————————————————————————————————-(ii)
Where, 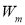 is the weight of lipid remaining,
is the weight of lipid remaining, 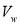 the volume of aqueous layer,
the volume of aqueous layer, 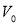 volume of solvent in each extraction step,
volume of solvent in each extraction step, 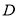 the distribution ratio of lipid in solvent,
the distribution ratio of lipid in solvent, 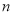 the number of extraction steps. The selection of solvent is then done using the distribution ratio of lipids in known solvents.
the number of extraction steps. The selection of solvent is then done using the distribution ratio of lipids in known solvents.
Folch method uses chloroform-methanol extraction followed by washing with water. This extracts all lipid from tissue except strandin which remains with the non-lipid phase. (Folch, 1957) Folch method was improved by Bligh and Dyer to improve the speed of extraction and purify the sample at the same time. (Bligh & Dyer, 1959) Extraction using low toxicity solvents like hexane: propanol has also been developed. (Hara & Radin, 1978)
Batch extraction is usually slow and requires a large amount of solvent. When other faster and easier methods are available, this method is not preferred. However, as no sophisticated equipment is necessary, batch extraction is very useful where the cost of equipment outweighs the usefulness of more accurate data.
3.1.2. Continuous Extraction
Continuous solvent extraction recycles the solvent used so that small amount of solvent can accomplish the equivalent extraction of several steps. This process is preferred for solid samples and sample where the distribution ratio is low. These samples need multi step extraction as very little lipid is extracted to the solvent in each step. (Pomeranz & Meloan, 1994)
Soxhlet extractor is widely used extractor for lipid. Although first developed to measure milk fat it has developed as a standard extractor for lipid as well as other substances. (Soxhlet, 1879) It has gone various improvements since its first publication and now various modifications are commercially available.
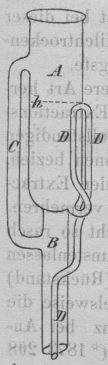
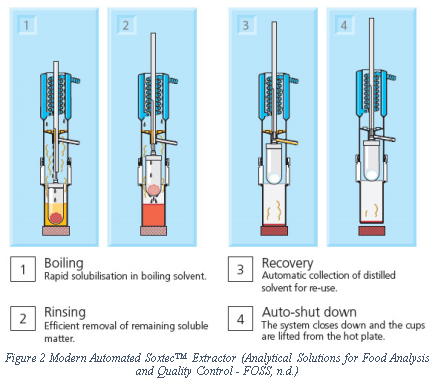
Goldfish extraction is a faster extraction system which suspends the sample in the solvent vapour. Although faster than Soxhlet based system, it might not completely extract the lipid due to channeling i.e. solvent may take a preferential path in the solid sample and may not cover whole of the sample. (Moreau, 2005)
As continuous extraction is faster and uses less solvents than batch extraction it is the most widely used extraction system. Moreover, the equipment used is not very expensive and can be used for extraction of other materials. However, these processes are slow and disposal of solvent is an everyday problem.
3.1.3. Pressurized Fluid Extraction
Pressurized fluid extraction (PLE) is carried out in high pressure and high temperature. In literature, this process is also called ‘Pressurized Solvent Extraction’ or ‘Accelerated Solvent Extraction’. The term ASE®is the registered trademark of Dionex Corporation which manufactures pressurized fluid extraction apparatus commercially. (Dean, 2009)
Richter et al. studied the effect of variables like temperature, pressure, solvent volume on extraction. Their work showed that the ASE® extractor could provide results comparable to Soxhlet but with reduced extraction time and solvent volume. This is attributed to decreased viscosity of solvent, weaker bond between components and increased diffusion capacity of solvent at higher temperature. Increased pressure is primarily applied to keep the solvent liquid, however, it provides the added advantage of forcing the solvent into pores blocked by insoluble matter. (Richter, et al., 1996)
However, there is evidence that PLE is not selective to lipids for certain foods. (Boselli, Velazco, Caboni, & Lercker, 2001) Moreover, no significant difference was seen on lipid extracted from poultry meat between Folch, Soxlet or ASE® method. (Toschi, Bendini, Ricci, & Lercker, 2003)
Pressurized solvent extraction can be highly useful in labs where routine extraction is required as it greatly reduces the extraction time and solvent use. On the other hand, the investment on the apparatus may not be economical if extraction is not carried out regularly. In all cases the stability of temperature sensitive components should be known before using PLE.
3.1.4. Supercritical Fluid Extraction
Substance in temperature above its critical temperature and pressure is called supercritical fluid. (IUPAC, 1997) Solvent property of supercritical fluid was first demonstrated in 1879. (Hannay & Hogarth, 1879) They have huge prospects in extraction because they combine the solubility power of liquid with penetration power of gas. Moreover, their solubility can be fine-tuned by changing the pressure and temperature. Carbon dioxide and water are the most promising fluid for supercritical extraction due to their non-toxicity and environmentally friendly nature. (Hedrick, Mulcahey, & Taylor, 1992)
3.2. Non-Solvent Liquid Extraction
In this method, the sample is treated with some liquid reagent which separates the lipid from sample and the lipid fraction is then measured. This method is mostly used to determine milk fat. They require specialized vessels for each method and cannot determine phospholipids. (McClements, 2003)
Several methods are present:
3.2.1. Babcock Method
3.2.2. Gerber Method
3.2.3. Detergent Method
3.3. Instrumental Methods
Different instrumental methods have been developed to determine the total lipid content of the sample. They rely on some physical properties that vary systematically with lipid concentration. (McClements, 2003)
Based on the property measured it is mainly of three types:
3.3.1. Measuring Bulk Properties
- Density: Density decreases as lipid content increases. This relation can be used to know the percentage of fat in a sample. (McClements, 2003)
- Electrical Conductivity: Conductivity decreases as lipid content increases. Thus, it can be used as fat concentration measure. (McClements, 2003)
- Ultrasonic Velocity: This is a fast and non-invasive method for fat content calculation. Amplitude and brightness analysis can be used to find the fat content and also distribution. (Abdul, N, Mohd, Abu, & Z, 2013) Moreover, attenuation(absorption) of ultrasound is linearly proportional to the amount of fat in the body. (Dukhin, Goetz, & Travers, 2013)
3.3.2. Measuring Absorption of Radiation
- UV-VIS: Fatty Acid absorb UV light proportional to its concentration. This method requires sample preparation to remove substances like proteins and hydrophobic peptides which interfere with the measurement. (Forcato, Carmine, Echeverria, Pecora, & Kivatinitz, 2005) Because of the extraction and dilution needed the process can be time consuming and labor intensive. (McClements, 2003)
- IR: Near infrared (NIR) spectroscopy is mainly used to analyze fatty acid content in food. (Mossoba, Azizian, & Kramer, 2012) Fat show strong absorbance at 5.74 mm which provides rapid and online fat composition measure. (McClements, 2003) The carbonyl absorption is the major reason for lipid’s NIR activity. This method requires intensive calibration with other approved methods hence is mainly used for routine analyses of large number of similar sample. (Greenfield & Southgate, Review of methods of analysis, 2003)
- NMR: NMR is also a non-destructive and fast method of total fat analysis. Although it requires a calibration curve, it is better suited than IR or UV because it can be operated by non-experts and the calibration curve is long lasting. (Oxford Instuments Molecular Biotools, 2010)
- X-Ray: Fat absorbs less X-Ray than lean meat. Hence, by building a proper calibration curve, fat content in meat can be determined by X-Ray absorption (McClements, 2003)
3.3.3. Measuring Scattering of Radiation
- Light and Ultrasonic Scattering: Light as well as ultrasound waves are scattered by oil droplets present in emulsions. The linear relation between concentration of droplet and light scattering can be used to measure total fat, provided no other interfering molecules exist. (McClements, 2003)
- X-Ray: Fat molecules show a sharp X-Ray scattering peak at 1.1 nm-1 while a water rich tissue shows a peak at 1.6nm-1. Thus, varying amount of fat can give a scattering profile which can be used for fat content determination. (Elshemey, 2011)
3.4. Other Methods
3.4.1. Solid Phase Extraction
3.4.2. Microwave Assisted Extraction
Total Lipid Concentration is a very simple data about food. As lipid contain diverse chemical species, in addition to total lipid, the type of lipid and their distribution also plays a major role to determine the purity, nutritional value, aesthetic look and taste. Hence, complete information about the type of lipids is necessary for scientist as well as legal bodies. (McClements, 2003)
Sample preparation is the most important part in most method to analyses lipids. As the lipid is extracted, care should be given to prevent the change of one form of lipid to another. Hydrolysis should be rigorously prevented as it reduces triglycerides and increases free fatty acids. Extraction should cause as less oxidation as possible. (Greenfield & Southgate, Review of methods of analysis, 2003) Extraction in chloroform, chloroform-methanol and hexane-isopropanol is preferred. Storage of sample in cold at -20oC is preferred. (AAFCO Lab Methods & Services Committee, 2014) The various method present have their advantages and drawbacks and the preferable method depends on the type of food and the type of lipid to me examined, (Greenfield & Southgate, Review of methods of analysis, 2003)
4.1. Chromatography
It is a very powerful tool for lipid analysis. It can give compete profile of the lipid molecules in the given sample. Chromatography separates the different components of lipid in fractions, these are then subject to spectrometric analysis which gives the molecular identity as well as relative concentration. IR, NMR and Mass Spectrometry are most commonly used. (McClements, 2003) These methods, although very reliable and comprehensive are very expensive due to the instrumental and reagent cost and is only carried out where complete molecular identification is required. (Greenfield & Southgate, Review of methods of analysis, 2003)
Three types are used:
- TLC: Thin Layer Chromatography is used to find concentration of different lipid groups. The TLC plate is prepared with suitable adsorbent and kept in proper solvent. A drop of sample is placed on one end and let to flow. The plate after separation to different fractions is compared to standard plates to identify the lipids. The spots can be analyzed further by GC, MS, NMR. (McClements, 2003) After the advent of HPLC, TLC use has decreased considerably. However, after the availability of pre-coated plates, the use of TLC for instant result is still carried out when there are few samples only. It is cheaper than HPLC. However, care during experimentation is required. (Christie, Thin-Layer Chromatography of Lipids, 2011) This method cannot be used to separate different types of phospholipids. (Zaima, Goto-Inoue, Adachi, & Mitsutoshi, 2011)
- HPLC: High Performance Liquid Chromatography is now a preferred method for lipid analysis. This is because it is more versatile than TLC and operates at room temperature, thus can be used to analyses labile groups that cannot be done using GC. (Christie, Thin-Layer Chromatography of Lipids, 2011)
- GC: Gas Chromatography is the preferred method for analysis of trans fatty acid. It can also be used for triglycerides and fatty acids; however, methylation is necessary. (Greenfield & Southgate, Review of methods of analysis, 2003) Fatty acids are non-volatile, hence before carrying out GC, the lipids are saponified and methylated to give Fatty Acid Methyl Esters(FAME) which are volatile and can be used for GC. (McClements, 2003)
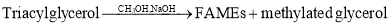 ——————————-(2)
——————————-(2)
It is now possible to convert a lipid sample of a fraction of a milligram in size to the methyl ester derivatives, separate these by gas chromatography, and have a quantitative result in under one hour. (Christie, Chapter 1 Introduction and Summary, 2011)
4.2. Chemical Methods
These methods are very cheap and do not require expensive machinery. However, only crude and average results are obtained.
Following test gives different information on fat:
- Iodine Value: It gives the average degree of unsaturation in the lipid. The lipid to be analyzed is titrated with ICl and the consumption of ICl gives the amount of unsaturation in lipid.
- Saponification Number: It gives the average molecular weight of triglycerols. The triglycerols are saponified with KOH and the amount of KOH used is determined. This is the saponification number. High saponification number corresponds to low molecular weight and vice versa.
- Acid Value: It gives the amount of free fatty acid. Here, the lipid is titrated with KOH until the solution turns alkaline. Other acids may interfere with results.
(McClements, 2003)
4.3. Instrumental Techniques
Various instrumental techniques for fat analysis are present. Methods like NMR, IR, MS are usually coupled with chromatography. Measurement of density and refractive index can be used to measure change in chain length and unsaturation. (McClements, 2003)
Lipids with high unsaturation undergoes aerial oxidation. This includes variety of reactions usually summarized as follows:
reactants � primary products � secondary products
(unsaturated lipids and O2) � (peroxides and conjugated dienes) � (ketones, aldehydes, alcohols, hydrocarbons)
5.1. Chromatography
Loss of reactants as well as formation of specific products can be monitored by using time profile.
5.2. Oxygen Uptake
Measures the amount of oxygen consumed over time while maintaining constant oxygen concentration on the reaction vessel.
5.3. Peroxide Value
Measures the amount of peroxide formed by titration with iodine.
5.4. Conjugated Dienes
Measures the concentration of conjugated dienes by UV spectroscopy (at 233nm for diene and 268nm for trines)
5.5. Thiobarbituric Acid(TBA)
Measures the secondary products (aldehydes) in the sample. The sample is treated with TBA and absorbance measured at 540nm. The absorbance value corresponds with the concentration of aldehyde.
5.6. Accelerated Oxidation Tests
The sample is oxidized in oxidation friendly environment and the time taken for rancidity to form is measured.
These tests help to know the physicochemical characteristics corresponding to flavor, appearance, flow etc.
6.1. Solid Fat Content(SFC)
Measures the fraction of fat present as solid. Density measure is mostly used
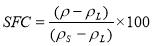 —————————————————————————————–(3)
—————————————————————————————–(3)
Where –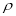 is the density at given temperature
is the density at given temperature 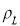 and
and 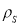 are the density if it was completely liquid or solid at the same temperature.
are the density if it was completely liquid or solid at the same temperature.
NMR signal decay rate is also being used recently. More solid component, faster is the signal decay.
Differential Scanning Calorimetry uses latent heat measure are also used to measure SFC-temperature profile.
6.2. Melting Point
Used when SFC is not required but only the temperature of melting is required. Due to different components present no sharp melting point is seen. Instead different melting points are used:
- Clear Point: The temperature at which fat completely melts and becomes clear
- Slip point: The temperature at which the fat in a capillary tube starts to slip.
- Wiley melting point: the temperature at which a disc suspended in alcohol-water mixture turns to a sphere.
6.3. Cloud Point
The temperature at which a completely melted lipid starts to develop turbidity.
6.4. Smoke Point
The temperature at which the lipid starts to smoke at standard condition
6.5. Flash Point
The temperature at which a flash appears on the surface at ignition at standard condition
6.6. Fire Point
The temperature at which a continuous flame stats to form at standard condition
6.7. Rheology
The measure of deformation and flow. Viscosity, elastic modulus and other relevant flow or plasticity measure is used.
(n.d.). (FOSS) Retrieved from Analytical Solutions for Food Analysis and Quality Control – FOSS: http://www.foss.dk/~/media/images/ca/soxtec8000/soxtech_extraction_sketch-jpg
AAFCO Lab Methods & Services Committee. (2014, January). Crude Fat Methods – Considerations. Retrieved from Association of American Feed Control Officials: http://www.aafco.org/Portals/0/SiteContent/Laboratory/Fat_Best_Practices_Working_Group/Crude_Fat_Methods_Considerations.pdf
Abdul, H. M., N, B., Mohd, S. M., Abu, K. R., & Z, M. (2013). The Use of Ultrasound As a Fat Measurement Sensor. International Conference on Smart Instrumentation, Measurement and Applications (ICSIMA), (pp. 315-320). Kuala LAmpur. doi:10.1109/ICSIMA.2013.6717974
Angelo, A. J. (1996). Lipid Oxidation in Foods. Critical Reviews in Food Science and Nutrition, 36(3), 175-224. doi:10.1080/10408399609527723
Bligh, E. G., & Dyer, W. J. (1959). A Rapid Method of Total Lipid Extraction and Purification. Canadian Journal of Biochemistry and Physiology, 911-917.
Bobe, G., Hammond, E. G., Freeman, A. E., Lindberg, G. L., & Beitz, D. C. (2003, October). Texture of Butter from Cows with Different Milk Fatty Acid Composition. Journal of Dairy Science, 86(10). doi:10.3168/jds.S0022-0302(03)73913-7
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


