Effect of Different Kinds of Fruit on Styrofoam Degradability
| ✅ Paper Type: Free Essay | ✅ Subject: Sciences |
| ✅ Wordcount: 2917 words | ✅ Published: 23 Sep 2019 |
Investigating the effect of different kinds of fruit on Styrofoam degradability
How can we degrade Styrofoam?
I will investigate on ‘How do different kinds of fruits affects Styrofoam degradable?’ by observing Styrofoam pieces which are affected by different kinds of fruit and fruit peel extracts. This investigation will be doing by dropping 5 drops of each extract on Styrofoam pieces that are squished several fruits and fruit peels. We will compare the results of the Styrofoam by measuring a mass of the Styrofoam. After this investigation, I will be able to support the how different kinds of fruit affects Styrofoam bio-degradable. By supporting it, I will be able to figure out a sustainable way for degradable Styrofoam.
Introduction
Our group decided to investigate about the topic because while we were searching about some basic ideas of our project, we knew that Styrofoam is non-biodegradable and it is made from a lot of countries across the world, then it is transferred to many landfills around the world after one use, and even transferred to oceans and beaches. Nowadays, it is a common thing that people get a huge Styrofoam box when they order a tiny product. We thought it is unsustainable that people using too much Styrofoam boxes which are not required that much, because those Styrofoam boxes are not only hard to be degraded, but they are also destroying nature because some Styrofoam is trashed in the beaches. Even when the Styrofoam is lurked in the landfills, it charges huge part of the landfills, and it releases methane gas which breaks ozone layer. According to greenliving.lovetoknow.com, polystyrene (Styrofoam) is non-biodegradable, and Cleveland State University states that it requires more than a million years to decompose. Furthermore, according to Scientific American, in 2014 a total of 28,500 tons of Styrofoam was produced, and 90% was used to make single-use cups, trays, containers and packaging products. But since they cannot be biodegraded, almost all of them are thrown away to the landfills and charges 25% of them, according to Ashton Cofer, who spoke at TED and was one of the winners of google science fair.To stop this vicious cycle of environmental destruction, we researched about Styrofoam decomposing and found a chemical called limonene that breaks down Styrofoam. It is from peels of citrus fruits like orange and lemon, so it is organic, eco-friendly, harmless and reliable. In our society, orange peels can be easily gotten by eating an orange. Sedaily.com stated that 5% of 495,000 tons of oranges are wasted. Using this huge number of fruit peels, Styrofoam can be easily degraded without any harmful and expensive chemicals. By investigating which fruits can break down the Styrofoam quickly, we can reuse peels of the fruits that people just throw away, and also there are a lot of beneficial social effects by decomposing tons of Styrofoam. For example, by reducing Styrofoam from the landfills, soil pollution will get better, methane emission is reduced, so destruction of ozone layer gets better, which protects us from harmful lights of the sun like UV. It is necessary to let Styrofoam be degradable, because every year, use of Styrofoam increases but old Styrofoam is still stocked in landfills. If there are more people throwing away their disposable cups which are made with Styrofoam in the beaches and oceans, billions of lives under water would be dangerous. Even right now, there are a lot of wastes trashed into the Pacific Ocean. To protect our planet Earth, we will find which fruit biodegrades the Styrofoam the most effectively and quickly.
Background research
Before looking up how Styrofoam can be biodegraded, Styrofoam is made from an expanded polystyrene foam (EPS). It is made from polystyrene, and polystyrene has two kinds of uses as EPS and XPS (Extruded polystyrene). Styrofoam is actually a brand’s name of an expanded polystyrene foam, and it is used to make products like plastic, packaging, Styrofoam and other disposable goods. Polystyrene was discovered in 1839 by Eduard Simon, an apothecary from Berlin. After he worked out for the polystyrene, a lot more scientists worked out about the topic and now we can finally use Styrofoam and plastic goods. It is naturally transparent, and it is made with almost 98% of air so it is buoyant and light.
To lower Styrofoam’s volume, firstly collect wasted EPS blocks, reduce volume by pressure on them. Greenmax apolo series can compact all kinds of polystyrene foam. After this, they are reused to make new products like small pellets.
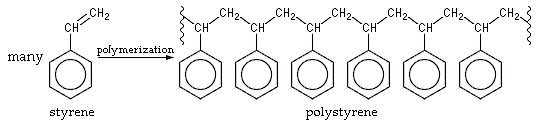
According to virtualscience.org, A chemical formula of polystyrene is (C8H8)n. Addition polymer of styrene result when vinyl benzene styrene monomers, which contain double bond between carbon atoms, attach to form a polystyrene chain, which has carbons that attached with a single bond to two other carbons and a phenyl group.
 Unfortunately, Styrofoam is so hard to dissolve that present recycling system cannot recycle it well, and there are massive number of polystyrene products all over the world. There is one way to dissolve Styrofoam and make the volume of it smaller. Virtualscience.com maintains that this is adding Acetone on it. Acetone dissolves huge amounts of Styrofoam with a small amount of it, so it can minimize the volume of Styrofoam.
Unfortunately, Styrofoam is so hard to dissolve that present recycling system cannot recycle it well, and there are massive number of polystyrene products all over the world. There is one way to dissolve Styrofoam and make the volume of it smaller. Virtualscience.com maintains that this is adding Acetone on it. Acetone dissolves huge amounts of Styrofoam with a small amount of it, so it can minimize the volume of Styrofoam.
Acetone is commonly used as nail polish remover, and it is an aprotic solvent with a large dipole moment that easily dissolves plastic. Acetone is more similar to plastic than other inorganic solids. However, using Acetone is not an eco-friendly way, as Acetone is toxic. At high concentrations, acetone vapor can cause CNS depression and cardiorespiratory failure and death. So new technology is needed to recycle Styrofoam in an eco-friendly way.
However, D-limonene can degrade Styrofoam with no further environmental problems. It is a major component of the oil of citrus fruit peels and it is a hydrocarbon which is classified as a cyclic monoterpene. It has the molecular formula of C10H16. Limonene is used in many ways of our society, such as beauty products, block cancer-forming chemicals and kill cancer cells in the laboratory, fragrance, cleaner (solvent), and as an ingredient in water-free hand cleansers. As it is a strong hydrocarbon, it also can be used to dissolve Styrofoam, as Acetone did. It also causes many positive effects in human body. According to WebMD, “D-limonene is a chemical found in the peels of citrus fruits and in other plants. It is used to make medicine. D-Limonene is used to promote weight loss, prevent cancer, treat cancer, and treat bronchitis. D-Limonene may block cancer-forming chemicals and kill cancer cells in the laboratory. But more research is needed to know if this occurs in humans.”
According to nothingsincurable.com, Grapefruit’s peels contain 88~95% of limonene, Tangerine peels contain 85~93%, Orange peels contain 85~96%, Mandarin peels contain 65~75%, lime’s contains 50~60% and Lemon peels contain 59~73%.
From research in Queen’s College, Limonene is a non-polar liquid covalent hydrocarbon, and Styrofoam is also a non-polar covalent hydrocarbon polymer. Since both two are non-polar substances, they are where the molecules have electrons that are distributed more symmetrically and therefore do not have a congregation of charges at the opposite sides and thus the charges all cancel out each other. They have weak intermolecular forces called Van Der Waals forces. Van Der Waals force is an attraction between temporary dipoles, and it is where there is temporarily changing electron density within molecules resulting in the formation of a temporary dipole which can induce a temporary dipole on another atom of another molecule. Non-polar substances cannot be dissolved in water and other polar, but they will be dissolved in non-polar substance. This is based on ‘like dissolves like’ rule, and it refers to polar and non-polar solvents and solutes. For example, if water is polar and oil is non-polar, water will not dissolve oil. Non-polar substances don’t dissolve in polar substance, because the attraction between the solvent molecules is stronger than the attraction between the nonpolar solute molecules. However, in non-polar solvent such as limonene, has similar strength with other non-polar solutes like Styrofoam allowing to have new interactions between the Styrofoam and limonene. Therefore, solute Styrofoam dissolves in the solvent limonene. The dissolution makes Styrofoam be degraded and collapse to a much lower volume since it is mostly made up of air. At the end, the product is a substance like glue which is sticky and stick things together.
In 2010, virtualsciencefair.org tried to biodegrade Styrofoam with limonene in different citrus fruit extracts and acetone. They tested their solutions which contain limonene and acetone on a Styrofoam cups. After their observations, they’ve got results of Styrofoam degraded from their solutions, and according to their results, orange and grapefruit are the most effective solutions among lime, lemon, orange and grapefruit, and lime didn’t have significant effect but still broke down some of Styrofoam. Moreover, they found that acetone and D-limonene both dissolve Styrofoam, and their time to dissolve same volume of Styrofoam is similar or solvent containing D-limonene takes quicker time to dissolve.
In 2015, Stanford’s researchers discovered that mealworms, also known as darkling beetle larvae, could digest and subsist youthfully on the diet of Styrofoam. It seemed it gives hopeful sign to our world, since microorganisms in the worm’s gut biodegrade polystyrene in the process. About 100 meal worms could consume between 34 and 39 milligrams of Styrofoam in a day. After eating Styrofoam, the worms were as healthy as the other worms which have normal diet, and their wastes were also safe that can be used as soil for crops.
Likewise, there were many people tried to dissolve Polystyrene in eco-friendly way, but at present, there are no representative to eco-friendly degrade Styrofoam and other polystyrene wastes or recycle them with machines that costs much.
Hypothesis
I predict that different kinds of fruit peel extracts would decrease mass of Styrofoam after dropping the extracts. Because the fruits I use for the investigation are all citrus fruits, their peels contain D-limonene in them, but amount of limonene in each fruit is different, so as much as a fruit contains more limonene, mass of the Styrofoam would decrease because of the degradation. I predict that grapefruit will degrade Styrofoam the most, because according to nothingsincurable.com, Grapefruit’s peels contain 88~95% of limonene, which has highest standard percentage as 88.5% among other citrus fruits. Tangerine peels contain 85~93% of limonene, Orange peels contain 85~96%, Mandarin peels contain 65~75%, lime peels contain 50~60% and Lemon peels contain 59~73%. Therefore, I consider that the sequence would be like: grapefruit peels, orange peels, Tangerine peels, Mandarin peels, lemon peels and lime peels, depending on how much limonene in each fruit.
However, I think that fruit extracts won’t be helpful to Styrofoam pieces to dissolve, because a chemical which degrades Styrofoam is limonene, and it is found in oil of citrus fruit peels. Since it is a strong hydrocarbon, it dissolves Styrofoam, as acetone did. Adding to that, I hypothesize that limonene in citrus fruit peels extract would cause Styrofoam to be bio-degraded, because Styrofoam and limonene are both non-polar substance which have Van Der Waal Forces, and it is an attraction between temporary dipoles, and it is where there is temporarily changing electron density within molecules resulting in the formation of a temporary dipole which can induce a temporary dipole on another atom of another molecule. Non-polar solvent such as limonene, has similar strength with other non-polar solutes like Styrofoam allowing to have new interactions between the Styrofoam and limonene. Therefore, solute Styrofoam dissolves in the solvent limonene. The dissolution makes Styrofoam be degraded and collapse to a much lower volume since it is mostly made up of air. At the end, the product would be like glue which sticks things together.
Variables
Independent Variable: Different kinds of fruit extracts
Dependent Variable: Mass of Styrofoam
Depend on different kinds of fruit extracts, mass of Styrofoam changes after an observation.
Control Variable
|
Control Variable |
How to Change this? |
Why? |
|
Number of drops on the Styrofoam pieces |
I will control this by dropping 5 drops of each fruit extracts on Styrofoam pieces |
It is important to control number of drops on the Styrofoam pieces, because by increasing number of drops, how much Styrofoam dissolved also increase. This means that later, when I measure each Styrofoam pieces after the observation, a one which was dropped 10 drops could weigh lesser than another one which was dropped 5. To prevent confusing my results to compare, I must keep our number of drops as 5 drops. |
|
Same Pipet |
I will control this by using only 1 pipet from the school’s Science lab. |
I must keep this same because similar to number of drops, different types of pipet might bring errors to my results. Although I drop 5 drops per each, bigger pipet can drop heavier amount of solution than smaller pipet, and this could make our investigation much harder to compare results. |
|
Same size of Styrofoam pieces |
I will control this by measuring mass of each Styrofoam by a scale of the Science lab and cut each Styrofoam pieces as 2*10cm with ruler and a paper clip. |
Same mass of Styrofoam is necessary for our investigation, because if there are two Styrofoam pieces which has large and small surface area, it could effect on Styrofoam degradability, because larger surface area can be dissolved better than the another. Also, mass of Styrofoam is also important, because I need to compare each results at the end of the investigation, so same mass of Styrofoam pieces will make my comparison easier. |
|
Same amount of time to wait for Observation |
I will control this by only waiting 1 minute for each fruit. |
I must keep this same because the different time of observation can make less mass or more mass of Styrofoam. This is because if the time is longer, it can cause a more chemical reaction than other fruits which means that Styrofoam will have more mass. |
|
Same type of Styrofoam |
I will control this by only using 1 big Styrofoam (EPS) board. |
I must keep this same because a different type of Styrofoam has different characteristics. Because of the different characteristics that different type of Styrofoam has, some Styrofoam might dissolve slower than other Styrofoam. |
|
Same Measurement |
I will control this by using the same measurement when measuring a mass (g) or a length (cm) |
I must keep this same because different measurement can be confused when analyzing results. Therefore, we will only use g when measuring a mass and cm for measuring a length. |
Equipment list
- 1 Orange – To test a solubility of Styrofoam
- 1 Lemon – To test a solubility of Styrofoam
- 1 Grapefruit – To test a solubility of Styrofoam
- 1 Lime – To test a solubility of Styrofoam
- 8 50ml Beakers – To put fruit extract
- 1 Pipet – To drop fruit extract on Styrofoam pieces
- 24 20cm2 (2*10) Styrofoam piece- To observe a solubility of Styrofoam and dissolve Styrofoam with a fruits extract
- Paper clip – To cut Styrofoam
- 1 Scale – To measure Styrofoam pieces
- 1 Bunsen Burner – To heat up the paper clip
- 1 Timer – To measure time to Styrofoam to be dissolved
Method
1. Prepared all the equipment that is needed in the experiment
2. Read through the risk assessment so we can avoid the risks
3. Created a result table which contains independent and dependent variables of x and y-axis
4. 8 Styrofoam pieces of 2*10cm size were measured with a ruler
5. A stretched paper clip was heated by a Bunsen burner
6. The clip was used to cut Styrofoam pieces
7. Measured Styrofoam pieces by a scale and recorded on the table
8. Peeled off the fruits
9. Squeezed the peels and the internal flesh into each beaker
10. Dropped 5 drops of each extract on the top of each Styrofoam piece
11. Measured 1 minute with a timer to observe
12. After a minute, mass of the Styrofoam pieces is measured and recorded on the result table
13. Repeated from step 4 to 12 for 2 times to make an accurate result table to support the hypothesis
14. Compared a completed result table and analyze
This method will help us to proceed the experiment progressively and effectively without making mistakes and missing out several steps. By using this method, we will wisely use our time to finish our investigation and there would be no further emergency conditions.
The method will support my hypothesis that
Sources Cited
A Student Discovered How to Seporate Limonene from Orange Peels. 서울경제, 3 Apr. 2018, www.sedaily.com/NewsView/1RY43KMGWX
A-Green-Way-to-Get-Styrofoam-Away. Studylib.net, studylib.net/doc/7209163/a-green-way-to-get-styrofoam-away
NAVER Knowledge. Ashton Cofer Invents a Way to Recycle Styrofoam, terms.naver.com/entry.nhn?docId=5696663&cid=51636&categoryId=63039.
2012 Virtual Science Fair Project, www.virtualsciencefair.org/2010/yoosxs2.
Hogue, Cheryl. “NYC Bans Expanded Polystyrene Food Containers, Opens Market to Alternatives.” Scientific American, 14 Jan. 2015, scientificamerican.com/article/nyc-bans-expanded-polystyrene-food-containers-opens-market-to-alternatives/
How to Recycle Polystyrene? – INTCO Recycling. EN-Main, www.intcorecycling.com/How-to-recycle-polystyrene.html.
Kinhal, Vijayalaxmi. “How Styrofoam Is Bad for the Environment.” LoveToKnow, LoveToKnow Corp, greenliving.lovetoknow.com/How_Styrofoam_is_Bad_for_the_Environment.
Martu, Sean. “D-Limonene Stops Acid Reflux (GERD).” Nothingsincurable, 9 Jan. 2019, www.nothingsincurable.com/d-limonene-cures-gerd/.
Plastic-Eating Worms May Offer Solution to Mounting Waste, Stanford Researchers Discover. Lack of Brain Protein Causes Sleeping Disorder Narcolepsy in Humans: 9/2000, 29 Sept. 2015, news.stanford.edu/pr/2015/pr-worms-digest-plastics-092915.html.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


