Effects of Acid Base Catalysis on Dry Yeast and Hydrogen Peroxide
| ✅ Paper Type: Free Essay | ✅ Subject: Sciences |
| ✅ Wordcount: 2714 words | ✅ Published: 15 Sep 2017 |
Abstract
The purpose of this experiment was to figure out if either acids or bases accelerate or decelerate the chemical reaction consisting of dry yeast and hydrogen peroxide. I am trying to prove that the more acidic or the more basic the reaction is, the more accelerated the reaction will be. Enzymes are very important to the human body because they speed up chemical reactions without being a part of it. Enzymes are made up of proteins which are important biological compounds in the formation of living organisms. The addition of an acid or base to yeast makes a certain amount of bubbles to show how acidity or basicness affects the chemical composition taking place in the reaction with the yeast. Without the addition of an acid or a base, the reaction is harmless to our bodies. The enzyme Catalase is used in everyday life as well. The protein found in the enzyme is easily changeable with the addition of another substance
Among the materials that you need to conduct this experiment are five clear containers, a washable spoon, distilled water, a measuring cup, baking soda, lemon juice, and a set of measuring spoons. The six planned concoctions are control with no acids or bases, low-acid with one teaspoon oflemonjuice, high-acid with two teaspoons of lemon juice, low-base with one teaspoon of baking soda, and high-acid with two teaspoons of baking soda. You might even want to try a combination of both the acid and the base. Next, you must add a Y4 cup of hydrogen peroxide into the glass. Then add a 1teaspoon of dry yeast and the reaction will begin. Record your results carefully to track this marvelous experiment.
In the end, the reactions that were further away from a neutral pH performed in a more decelerated rate. Therefore, the control, low-acid, and low-base reactions performed at a more accelerated rate than the high-acid and the high-base reactions. However, the combination reaction performed at an exponentially better rate than all other reactions. Although all the mixtures performed within the same range (besides the combination), it was simply due to the reactions being at a microcosmic scale.
The experiment ended up proving my initial hypothesis completely incorrect. It would probably be a wise idea to use larger amounts in order to get more appreciable results. The bubbles formed because different atoms in the hydrogen peroxide and the dry yeast collided and then bounced away to be farther away than they were in the beginning. This microscopic change appears to us humans in the form ofbubbles.
The enzyme Catalase found in dry yeast, is also found in our bodies’ organs; primarily the liver. What Catalase does in the liver is manage the graying of our hair. The more Catalase the faster our hair will gray, and the less Catalase there is the slower our hair will gray. Since Catalase is found in our crucial organs, doctors and scientists have done experiments to try and manipulate the enzyme. Their experiments primarily consist of the yeast acting against acids and bases as I did in my project. If this experiment were to be done on a more grand scale, it would sure affect and aid us in our everyday lives.
Introduction
The purpose of this project is to figure out if either acids or bases accelerate or decelerate the chemical reaction consisting of dry yeast and hydrogen peroxide. Enzymes are very important to the human body because they speed up chemical reactions without being a part of it. This catalysis isn’t just found in the human body, it’s also in most living things on Earth. Enzymes are made up of proteins which are important biological compounds in the formation of living organisms. The addition of an acid or base to yeast makes a certain amount of bubbles to show how acidity or basicness affects the chemical composition taking place in the reaction with the yeast. If you have ever mixed baking soda and lemon juice in an attempt to fight indigestion, you will see a basic chemical reaction between the two. Without the addition of acids or bases, the yeast reaction is quite harmless to our bodies. However, since we consume acids and bases almost every day, it’s a great idea to enlighten yourself on just how our bodies are working. The main goal of this experiment is to fmd out how well the catalase in yeast breaks down acids and bases or vice versa.
Hypothesis and Background Research
Acids and basses are two very common terms in many scientific fields, such as chemistry. Acids are chemical substances that dissolve some types of metal and turn litmus intro a red color because of them being of a pH lower than seven. They are typically a corrosive or sour-tasting kind of liquid. Bases on the other hand, are usually of a pH higher than seven and are the opposite of acidic substances. They accept hydrogen ions instead of releasing them such as acids do. Bases will also typically turn litmus paper into a sort of blue color.
There are several different types of chemical reactions and changes happening around us in our everyday lives. The most common of these reactions occurs when a raw egg turns solid. This happens because an impressive amount of heat is applied to the raw egg which forms longer and stronger chains of protein molecules inside the egg. This reaction and several others that occur in our body rely on enzymes, which are basically special types of catalysts made up of protein. Catalysts are anything that speeds up an action without being used up themselves. Thus, an acid- base catalysis is the acceleration of a chemical reaction by the addition of an acid or a base with the acid or base itself not being consumed in the reaction. Enzymes are not only found in human bodies, they are found in all types of living things including yeast. Yeast contains the enzyme known as catalase which breaks down the chemical hydrogen peroxide (H202) into oxygen gas and water. This would be the reaction that will inform us about the amount of bubbles formed from the acids and bases. This reaction will also show us how much the yeast has to work to break down the hydrogen peroxide when different substances are also added onto the concoction. Proteins can be changed when a specific amount of heat is brought upon it. Since enzymes are made up of proteins, they too can be changed by heat. However, what a majority of people do not know, is that the addition of acids and bases can also affect the way that a protein is put together.
Both acid-catalysis and base-catalyzed reactions are used for their own unique purposes. A macrocosmic example of acid catalysis is the reaction and conversion of the hydrocarbon atoms found in petroleum to gasoline, and the creation of silicone. An example of a grand base catalyzed reaction is the creation and conversion of several compounds and molecules used in the creation of foam sponges.
The main reasoning behind this investigation is to discover how well the catalase enzyme in yeast can break down hydrogen peroxide after different amounts of acids and bases have been added onto it. For this experiment, my hypothesis is that the more acidic or the more basic the concoction made in the different cups is, the more bubbles will be made and the higher they will get.
Procedure
Materials
The materials you will need for this experiment include:
- 5 clear glass containers of equal size (beakers or test tubes are ideal)
- Permanent marker
- Tape
- 5 clean spoons
- Distilled water
- Small clear cup/glass
- Baking soda
- Set of measuring teaspoons
- Measuring cup
- Hydrogen peroxide
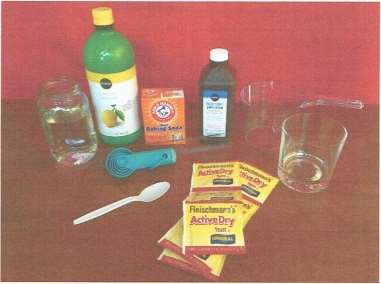
- Dry yeast
- Ruler
- Lemon juice
Variables
- The rlcpcndent-ormanipulated variable in this experiment is the amount of lemon juice or baking soda poured into the different containers and thus, the acidity or basicity in each container.
- The iodepor responding variable in this experiment is the height and amount of bubbles formed as a result of the chemical reaction.
- The controlled variable or the variable held constant in this experiment is the amount of yeast and the amount of hydrogen peroxide put in each container and the containers themselves.
Step–by–Step Directions
- Label the containers: 1- Control, 2- Low Acid, 3- High Acid, 4- Low Base, and 5-High Base.
- Put a spoon in each of the containers, and make sure to never move a spoon from one container to the other.
- Add two teaspoons of distilled water to container 1- Control.
- Stir in 1 4 cup of hydrogen peroxide to container 1-Control.
- Stir in 1 teaspoon of yeast to container 1- Control.
- Place the ruler alongside the container, and record the highest height the bubbles reach
- Of the other containers, record predictions first, and actual results after on a chart.
- To create the acidic containers, add one teaspoon oflemonjuice to container 2- Low Acid and two teaspoons oflemonjuice to container 3-High Acid.
- Add one teaspoon of distilled water to container 2- Low Acid so it is the same volume as con iner 3.
- Stir in V4 cup of hydrogen peroxide to containers 2 and 3.
- Add 1 teaspoon yeast to both container 2 and 3. Stir and observe.
- Record the maximum height ofthe yeast bubbles.
- To create the basic containers, add one teaspoon ofthe baking soda solution to container
- Low Base and two teaspoons of the baking soda solution to container 5- High Base.
- Add one teaspoon of distilled water to container 4- Low Base so it has the same volume as container 5.
- Stir in V4 cup ofhydrogen peroxide to containers 4 and 5.
- Add 1 8 teaspoon of yeast to both container 4 and 5. Stir and observe
- Record the maximum height ofthe yeast bubbles. (Compare your predictions with your actual observations)
Results
There were a plethora of things to be discovered from this otherwise simple experiment. The very flrst thing that you have to be aware of to do this experiment is that there will always be a change to an altered chemical reaction, no matter how small the alteration or the result. The result of each and every chemical reaction wasn’t very different, but it was enough so that each showed a noticeable change. The temperature for each experiment I conducted stayed at approximately the same level throughout. The original height of the mixture was approximately 1 inch before adding the yeast. I performed three separate trials for each chemical reaction. The results were approximately the same for every trial I conducted of the different concoctions. The initial height of all the concoctions prior to adding yeast was approximately 1 inch.

The constant reaction worked at the most accelerated rate, thus causing more bubbles to form on the mixtures surface. This occurred because of the fact that the enzyme Catalase works best at around pH 7, and this mixture was very near to the neutral pH. The foamy bubbles made the height of the concoction reach approximately 1.5 inches in an average whiskey glass. The bubbles reached their maximum height at a slow rate. This was true for a majority of the reactions.
Control Reaction
The acidic reactions reacted in a very similar way to each other. The low-acid reaction acted in a very similar way to the control reaction in every single trial I conducted. The bubbles in this reaction reached a slightly lower height than that of the control reaction; approximately 1.2 inches. The pH of this composition was slightly more acidic; about a 6 or 5 on the pH scale. The pH being lower is what caused the bubbles to perform in a more decelerated rate. The high-acid reaction also performed at a lesser magnitude than the control reaction. The height of the bubbles reached a height of slightly more than 1 inch. Due to the fact that the high-acid reaction had a lower pH and strayed further from the desired neutral status, it performed the worst of all the reactions thus far. However, this reaction reached its maximum height in a shorter amount of time.


Low-Acid Reaction High-AcidReaction
The low-base mixture reacted in approximately the same way as the low-acid mixture. This is because the two mixtures were the same amount of pH away from the desired neutral pH. This concoction was at a pH of roughly 9 or 10. The height ofthis mixture was approximately 1.2 inches. Even though the amount of acid or base added to the mixture was the same, the one teaspoon of baking soda raised the pH more than the one teaspoon of lemon juice lowered the pH because the baking soda is a powder. It being a powder allows for the individual molecules of the substance to spread around the mixture more than the tangy lemon juice could. The high-base mixture reacted in a very similar way to the high-acid mixture. Again, this was because they were the same amount away from a neutral pH. The pH of the high-base concoction was a pH of approximately 11 or 12. The maximum height of this mixture reached slightly more than 1 inch. This blend also reached its maximum height in a shorter amount of time than the others.


Low-Base Reaction High-Base Reaction
Due to the fact that the different reactions reacted in quite a similar way to one another, I decided to conduct an additional experiment. This one consisted of one teaspoon of lemon juice and one teaspoon of baking soda in the beginning. This was to discover if a mixture of the two would accelerate or decelerate the Catalase reaction. I had previous knowledge that a mixture of baking soda and lemon juice resulted in a foamy liquid that helped with indigestion and to fight off minor cancer cells, so I put it to the test with the catalytic enzyme. This concoction reacted in a way like no other. The maximum height of the reaction was approximately 5 inches. This reaction also reached its maximum height quicker than any other reaction. The initial foam of the mixture of the acid and the base caused the yeast bubbles to be larger and whiter in color in comparison to the other reactions.

|
Estimated Height of Yeast Bubbles |
Actual Height of Yeast Bubbles |
|
|
Control |
2in. |
1.5 in. |
|
Low-Acid |
3 in. |
1.3 in. |
|
High-Acid |
4in. |
1.1in. |
|
Low-Base |
3.3 in. |
1.35 in. |
|
High-Base |
4.4in. |
1.15 in. |
|
Combination |
5 in. |
2.5 in. |
Conclusion
The results proved my hypothesis completely incorrect. I believed that the further away from neutral the concoctions got, the more accelerated the reaction would be. However, the complete opposite to what I believed turned out to be true. I was very surprised to see that every planned reaction gave approximately the same results. That was why I decided to conduct an experiment with usually counteracting substances; the acid and the ba::}if I were to do this experiment again, I would use larger amounts in order to get larger and more visible results. The most plausible explanation of the yeast reaction is that the bubbles formed because the hydrogen and oxide atoms collided with the Catalase in the yeast and then bounced away. Due to the fact that the molecules bounced apart, a larger microscopic gap formed between the atoms. The way us humans see this minuscule separation is in the form of the Catalase bubbles. The way that this reaction could help us in our everyday lives is actually quite simple. Catalase is found in a majority of human bodies; especially in the liver. What Catalase does in the human body is that if there is more of it in the liver, your hair will gray at a slower rate or not at all, and if there is not a lot of Catalase in your liver, then your hair will grow at an exponential rate. Due to the fact that Catalase is found in one of our crucial organs, doctors and scientists have conducted experiments to try and manipulate the enzyme in order to treat ailments in that region of the bodese experiments were simply on a microcosmic scale, which did not allow them to perform in such a notable and appreciable way. However, on a larger scale, this type of catalysis would be truly helpful in our everyday needs.
Bibliography
Gray, Theodore. Molecules:TheElementsandtheArchitectureofEverything.New York
City: Black Dog & Leventhal, 2014. Print.
Touchette, Betty. (2014, May 01). Acid–BaseCatalysis.
Goodsell, David. (2004, September). PDB–101:Catalase
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


