Determination of Partition Coefficient
| ✅ Paper Type: Free Essay | ✅ Subject: Sciences |
| ✅ Wordcount: 1216 words | ✅ Published: 05 Apr 2018 |
Introduction
Diffusivityordiffusion coefficientis a proportionality constant between themolar flux due to moleculardiffusion and the gradient in the concentration of the species (or the driving force for diffusion). Diffusivity is encountered inFick’s law and numerous other equations ofphysical chemistry. It is generally prescribed for a given pair of species. For a multi-component system, it is prescribed for each pair of species in the system.The higher the diffusivity (of one substance with respect to another), the faster they diffuse into each other.This coefficient has anSI unit of m2/s (length2/ time). In CGS units it was given in cm2/s.
In this experiment, agar gel is used as the diffusion medium. The gel consists of the semi-solid network of agar molecules interpenetrated by water which form the continuous phase for diffusion. The agar molecular network prevents the speed up of water molecules movement which occurs naturally such as through the convection flow of water caused by temperature difference.
Objective:
- To determine the D value (diffusion coefficient) at different temperature, concentration and solvent/medium.
- To determine the radius and volume of the particle.
- To determine the mass of particle and molecular weight of substance
Methodology
- Molten agar solution is prepared by boiling the powder in an appropriate solvent.
- 200 mL 2% agar in Ringer solution.
- 50 mL 1% agar in Ringer solution.
- 50 mL 2% agar in Ringer solution.
- 10 mL of the molten agar is poured into specified test tubes and left to solidify.
- 2% agar in Ringer into 14 test tubes.
- 1% agar in Ringer into 2 test tubes.
- 2% agar in distilled water into 2 test tubes.
- The reference mixtures is prepared for Crystal Violet and Bromothymol Blue by mixing:
- 5 mL of the molten 2% agar in Ringer with 5mL, 1 in 500,000 Crystal Violet solution.
- 5 mL of the molten 2% agar in Ringer with 5mL, 1 in 500,000 Bromothymol Blue solution.
- 5 mL of the molten 1% agar in Ringer with 5mL, 1 in 500,000 Crystal Violet solution.
- 5 mL of the molten 2% agar in distilled water with 5mL, 1 in 500,000 Crystal Violet solution.
- 3 mL of the following solution is filled into specified test tubes containing the solidified agar. Each test tube is covered with aluminium foil and the time is noted.
- 1 in 200 Crystal Violet into 4 tubes containing 2% agar in Ringer.
- 1 in 400 Crystal Violet into 4 tubes containing 2% agar in Ringer.
- 1 in 600 Crystal Violet into 4 tubes containing 2% agar in Ringer.
- 1 in 400 Crystal Violet into 2 tubes containing 1% agar in Ringer.
- 1 in 400 Crystal Violet into 2 tubes containing 2% agar in distilled water.
- 1 in 400 Bromothymol Blue into 2 tubes containing 2% agar in Ringer.
- Test tube 4(i), (ii), (iii) is kept in an incubator at 370C. The rest of the test tubes is kept at room temperature.
- The distance between the origin (the solution or gel interface) to the point of a known concentration (the point where the colour is equivalent to the appropriate reference mixture) is measured accurately at 900s, 1800s, 2700s, 3600s, 4500s, 322200s and 415800s.
- The result is recorded and tabulated.
Results
a) 1 in 200 Crystal Violet (CV) in 2% agar in Ringer at room temperature.
Tube 1


Tube 2


b) 1 in 200 CV in 2% agar in Ringer incubated at 37°C
Tube 3


Tube 4


c) 1 in 400 CV in 2% agar in Ringer at room temperature.
Tube 5


Tube 6


d) 1 in 400 CV in 2% agar in Ringer incubated at 37°C
Tube 7


Tube 8


e)1 in 600 CV in 2% agar in Ringer at room temperature.
Tube 9


Tube 10


f) 1 in 600 CV in 2% agar in Ringer incubated at 37°C
Tube 11


Tube 12


g) 1 in 400 CVin 1% agar in Ringer at room temperature.
Tube 13


Tube 14


h) 1 in 400 CV in 2% agar in distilled water at room temperature.
Tube 15


Tube 16


i) 1 in 400 Bromothymol blue in 2% agar in Ringer
Tube 17


Tube 18


Calculation
Calculations were conducted to determine:
- the D value,
- the radius of the molecule,
- the volume of the particle,
- the mass of the particle, and
- the molecular weight of the substance
Equations used,
1. To determine the D value

*of which, x2 is y and 2.303(4)(D)(t)(log10m0-log10m) is (mx+c)
Representing the equation of straight line y=mx + c. In calculating the D value, the value of c (y-intercept) = 0, thus the new equation isy=mx.
Based on 2 equations to find the slope of the line, m;

and

D value is calculated as such,



Modified to,

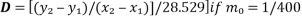


2. To determine the radius of the molecule 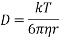

The radius is calculated as such,



3. To determine the volume of the particle

4. To determine the mass of the particle


5. To determine the molecular weight of the substance
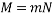


a) 1 in 200 Crystal Violet (CV) in 2% agar in Ringer at room temperature.
Tube 1


1. Determining D value



2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


Tube 2


1. Determining D value



2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


b) 1 in 200 CV in 2% agar in Ringer incubated at 37°C
Tube 3


1. Determining D value



2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


Tube 4


1. Determining D value



2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


c) 1 in 400 CV in 2% agar in Ringer at room temperature.
Tube 5


1. Determining D value

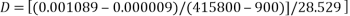

2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


Tube 6


1. Determining D value

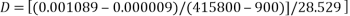

2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


d) 1 in 400 CV in 2% agar in Ringer incubated at 37°C
Tube 7


1. Determining D value



2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


Tube 8


1. Determining D value



2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


e) 1 in 600 CV in 2% agar in Ringer at room temperature.
Tube 9


1. Determining D value



2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


Tube 10


1. Determining D value



2. Determining the radius of the molecule

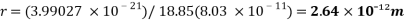
3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


f) 1 in 600 CV in 2% agar in Ringer incubated at 37°C
Tube 11


1. Determining D value


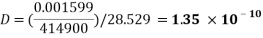
2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


Tube 12


1. Determining D value


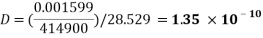
2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


g) 1 in 400 CV in 1% agar in Ringer at room temperature.
Tube 13


1. Determining D value



2. Determining the radius of the molecule


3. To determine the volume of the particle


4. To determine the mass of the particle

5. To determine the molecular weight of the substance


Tube 14


1. Determining D value


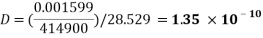
2. Determining the radius of the molecule
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal



