Mechanisms of Brain Development
| ✅ Paper Type: Free Essay | ✅ Subject: Physiology |
| ✅ Wordcount: 2155 words | ✅ Published: 18 May 2020 |
Mechanisms of Brain Development
many studies have been conducted before to investigate the mechanism behind the development of the optic pathway, understanding has been limited, especially in terms of retinal ganglion cells (RGCs), a mediator cell in the visual pathway. A better understanding of this would not only benefit scientifically, but more importantly a possible benefit clinically, for instance detecting a defect on the molecular level underlying a disease. This study, thus, aims to identify the role of Dscam in the development and growth of optic ganglion cell axons.
Why is RGCs studied?
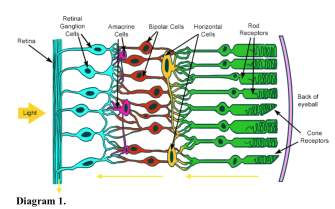 The pathway of how light enters our eyes and eventually reach our visual cortex involve multiple connections. Diagram 1 illustrates a brief summary of how photoreception works with the yellow arrow indicating the direction of signals, while RGCs further reach the lateral geniculate body of the thalamus and to the visual cortex. RGCs can be categorized into groups. Parvocellular (P) cells are responsible for colour discrimination, while magnocellular (M) cells mainly detect movement of stimulus and the form of objects. On-centre and off-centre ganglion cells increase the contrast at light-dark borders of images. It is important to note the role of retinal ganglion cells as the only neuron whose axons extends out of the retina.
The pathway of how light enters our eyes and eventually reach our visual cortex involve multiple connections. Diagram 1 illustrates a brief summary of how photoreception works with the yellow arrow indicating the direction of signals, while RGCs further reach the lateral geniculate body of the thalamus and to the visual cortex. RGCs can be categorized into groups. Parvocellular (P) cells are responsible for colour discrimination, while magnocellular (M) cells mainly detect movement of stimulus and the form of objects. On-centre and off-centre ganglion cells increase the contrast at light-dark borders of images. It is important to note the role of retinal ganglion cells as the only neuron whose axons extends out of the retina.
The foundation of the study is that axonal growth and orientation is not simply programmed, but relies on the external environment, i.e. extrinsic cues. An experiment that studied about the pathway of Mauthner cell showed this characteristics of axonal development (Hibbard, 1965). A number of cues that interact with the growth cone of the axon, for instance those produced by intra-fascicular glial cells, can either be attracting or repelling. Growth cones then have the ability to navigate and branches in response to the external cues. Cytoplasmic projections from the growth cones, which are called filopodia, eventually binds to certain extracellular matrix components and hence stimulates actin filament assembly and motility, allowing for axonal growth.
A number of these molecular cues have already been identified, for instance laminin and vitronectin, which promote axonal growth. However, different neurons respond differently to extracellular cues. For example, CNS neurons respond better to laminin while PNS neurons respond to fibronectin better. This suggests why this study specifically of RGCs mechanism was conducted. Currently, mechanisms regarding RGC differentiation and axonogenesis is quite well-known. In addition, known mechanisms of related to RGCs growth and navigation out of the retina, crossing at optic chiasm and through optic tract include promoting factors such as Vax 1 protein, NrCAM, FGF-2; inhibitory factors such as Slit, β1-integrin/N-cadherin, Shh (1). The inhibitory mechanisms is certainly better established. As all these contribute to normal visual function, it is worth studying deeper into the mechanism of RGC development.
Hence, the author has identified Down syndrome cell adhesion molecule (Dscam) as a possible promoter in the process. Dscam has previously been shown to play a crucial role in neural circuit formation of Dosaphilia and certain functions in mouse visual system including promotion of self-avoidance and regulation of retinogeniculate refinement. Nevertheless, the fact that it is expressed in early embryonic stage may indicate its other functions. The author hypothesize that Dscam acts as a cue in the promotion of axon fasciculation and axonal growth of RGCs.
Methods of Investigation
To investigate the roles of Dscam, six experiments have been conducted. Comparisons between Dscam wildtype mouse and mouse with mutant and overexpression of Dscam genes respectively were made to draw conclusions. Mutant Dscam gene is done by genetical engineering, inactivating gene expression by deleting the Dscam 17 genes. The chromosomal location and sequence of Dscam 17 gene is first identified, followed by the tailor-making of specific targeting vector that is used to inactivate or mutate the target gene in mouse embryonic stem cells. These cells are later injected into a mouse embryo so they grow with Dscam 17 mutant genes. In the experiments, axonal development was tracked by DiL staining. DiL is a vital fluorescent stains, which is able to enter the neuron membrane and diffuse down the axonal processes due to its lipophilic property, thus labelling the neuron evenly. It is then be visualized with epifluorescent illumination. It can be applied in both anterograde and retrograde labelling.
Mouse was used because of several advantages, with the most significant reasons being that it is highly genetically tractable, it is possible and simple to mutate selected genes, and most importantly that mouse’s genome has high resemblance to human’s one. These properties allow investigation to be of high value as the chromosomal location and expression of the genes in human should be very close.
Key Experimental Findings
The experiments study the location of Dscam and investigate the functionality of Dscam in axonal growth and fasciculation by comparing Dscam del17/del17 and DSCAM wildtype mouse in terms of morphology of axons, direction of axon growth, number of axons, size of axons and morphology of growth cones. The key findings are as follows:
This validates the fact that Dscam is expressed at a very early stage and provide the basis for this study. |
This shows Dscam as a separate entity from netrin-1, which is essential for RGC axon growth out of the eye (Deiner et al., 1997). |
This suggests the possible role of Dscam in RGCs axon growth in the optic tract. However, RGCs growth does occur in a progressive manner as the knockout mouse embryo grows, only comparatively shorter than that of wildtype mouse. This shows that there are other cues that mediate RGCs axon growth and the experiment should be carried on for a longer period of time to investigate whether other molecular cues could compensate the function of Dscam in RGC axon growth. |
This suggests that Dscam is Dscam is essential for RGC axon growth and fasciculation, which is the process of formation of groups of axons called bundles. |
This suggests Dscam’s intracellular role in promoting pre-synaptic axon growth in RGCs.
Culturing of Dscam +/+ explants with Dscam-producing cells induced a significant increase in the extent of RGCs axon outgrowth compared with cultures containing control cells. This suggests Dscam’s role as an extracellular promoter of RGC axon outgrowth.
This confirms results from previous experiments that Dscam’s act as both intracellular drive and extracellular cue in promoting RGCs axon growth. |
This suggests more Dscam would lead to more exuberant outgrowth of RGC axons. However, this does not explain how diseases like Down Syndrome, in which Dscam is overexpressed, will lead to visual problem given there is increased instead of decreased axon growth. |
These findings consistently show that there is a direct correlation between the molecule DSCAM and the stimulation of axonal growth and fasciculation of retinal ganglion cells, which supports the author’s hypothesis.
Significance of the Study
The study provides further information to the mechanism of development of RGC and optic pathway, which helps in identifying the underlying mechanism of visual defects in diseases. For example, Down syndrome patients have serious visual acuity problems including refractive errors such as myopia, nystagmus and strabismus. While these clinical features may be a result of structural defect of the eye, the overexpression of Dscam due to trisomy 21 may also cause the problems, considering the fact that these symptoms arise at early stage of life in Down Syndrome patients. For fragile X-syndrome, which is a X-linked defect caused by decreased or absence of FMR-protein, there is also an overexpression of Dscam due to mRNA mis-regulation. These features suggest possible role in how alteration in Dscam expressions lead to clinical features, and indicate possible direction for studying treatment methods. However, as this study concludes that Dscam gain of function results in exuberant growth into the dorsal thalamus, it may not explain the visual defect in Down syndrome patients. In addition, Fragile X patients mainly suffer from mental retardation and cognitive functions, which is not relevant to problems with the visual pathway. Dscam’s significance remains low for fragile X syndrome. There are also limited diseases that are shown to have direct association to Dscam.
Nevertheless, by integrating the conclusion of the study, which is the positive association between Dscam and RGC axonal growth, into current understanding of the development of RGCs, scientists and clinicians have more cellular and molecular mechanisms to look into to identify pathophysiology of diseases, especially in newborns. Screening of corresponding genes may also be performed in the future.
Limitations of the study
Regarding biological plausibility, the exact molecular level mechanism of how Dscam affects the growth and fasciculation of RGC, for instance the receptors or ligands that interact with Dscam, has not been identified. This is essential for treatment development
Regarding study methodology, some experiments are conducted in vitro. With the complexity of in-body architecture and organization, certain environmental factors and possible compensatory mechanisms may be lost in vitro. Culture condition may be depleted of nutrients and oxygen. Cell density may not be comparable to body conditions. These all may alter the conclusion as physiological response may be altered.
Regarding the experimental results, there may be possible alternative explanations. For example, as comparison between mutant and wildtype phenotypes is made on the same embryonic day, it is possible that absence of Dscam only slows down the axon growth process if Dscam only acts as a modulator or catalyst of another molecular cue. Although results from all experiments are highly consistent, there may be certain degree of bias in drawing conclusion right from experiment results.
Further Research
More studies can be conducted to complement this study and improve the significance of Dscam. Studying the axon growth pattern for longer period of time can improve the strength of the conclusion. A larger base of subjects can be studied to prove consistency of findings. Experiments may also be extended to study the growth and fasciculation of other neuron types, which may be give higher clinical significance in addition to the understanding of axon growth mechanism.
In fact, there are a number of studies which have developed this study further. It is found that Dscam differentially modulates pre- and postsynaptic structural and functional central connectivity during visual system wiring the developing retinotectal circuit (5). It shows Dscam’s role in the coordination of axons and dendritic arbors in embryonic development. Dscam is also studied in adults to investigate there is any difference. It was found to have an inhibitory function instead, inhibiting circuit-level plasticity (7).
To conclude, although this study has multiple limitation and limited significance, it is important in helping scientists understand the visual growth mechanism. More studies should be conducted in the future based on the crucial information in this study.
References
- Erskine, L., & Herrera, E. (2014). Connecting the Retina to the Brain. ASN Neuro, 6(6), ASN Neuro, 03 December 2014, Vol.6(6).
- Hattori, D., Millard, S., Wojtowicz, W., & Zipursky, S. (2008). Dscam-Mediated Cell Recognition Regulates Neural Circuit Formation. Annual Review of Cell and Developmental Biology, 24(1), 597-620.
- Neuroscience: Exploring the Brain (Bear, Connors, Paradiso)
- Roizen NJ, Mets MB, Blondis TA. Ophthalmic disorders in children with Down syndrome. Dev Med Child Neurol 1994; 36:594.
- Santos, R., Fuertes, A., Short, G., Donohue, K., Shao, H., Quintanilla, J., . . . Cohen-Cory, S. (2018). DSCAM differentially modulates pre- and postsynaptic structural and functional central connectivity during visual system wiring. Neural Development, 13(1), 1-19.
- “Scientists Can Analyze Gene Function by Deleting Gene Sequences”. Retrieved from https://www.nature.com/scitable/topicpage/scientists-can-analyze-gene-function-by-deleting-6526138/
- Simmons, A., Bloomsburg, S., Sukeena, J., Miller, C., Ortega-Burgos, Y., Borghuis, B., & Fuerst, P. (2017). DSCAM-mediated control of dendritic and axonal arbor outgrowth enforces tiling and inhibits synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America, 114(47), E10224-E10233.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


