Effect of Fat: Assessment of Apparent Diffusion Coefficient
| ✅ Paper Type: Free Essay | ✅ Subject: Physiology |
| ✅ Wordcount: 3811 words | ✅ Published: 11 Sep 2017 |
Abstract
Objectives: Recent studies have indicated that excessive fat may confound assessment of diffusion in organs with high fat content, such as the liver and breast. However, the extent of this effect in the kidney, which is not considered a major fat deposition site, remains unclear. This study tested the hypothesis that renal fat may impact DWI parameters, and proposes a three-compartment model (TCM) to circumvent this effect.
Methods: Using computer simulations, we investigated the effect of fat on assessment of apparent diffusion coefficient (ADC), intravoxel incoherent-motion (IVIM) and TCM-derived pure-diffusivity. In domestic pigs fed a high-cholesterol (Obese) or normal diet (Lean) (n=7 each), DWI parameters were calculated using IVIM and correlated to renal histology. IVIM-derived pure diffusivity was also compared among 15 essential hypertension (EH) patients classified by BMI (high vs. normal). Finally, pure diffusivity was calculated and compared in 8 patients with atherosclerotic renal artery stenosis (ARAS) and 5 healthy subjects using IVIM and TCM.
Results: Simulations showed that unaccounted fat results in the underestimation of intravoxel incoherent-motion (IVIM)-derived pure-diffusivity, particularly at lower fat contents. Moreover, TCM, which incorporates highly diffusion-weighted images (b>2500s/mm2), could correct for fat-dependent underestimation. Animal studies confirmed lower ADC and pure-diffusivity in Obese vs. Lean pigs with otherwise healthy kidneys. Similarly, EH patients with high BMI had lower ADC (1.9 vs. 2.1×10-3 mm2/s) and pure-diffusivity (1.7 vs. 1.9×10-3mm2/s) than those with normal BMI. Pure-diffusivity calculated using IVIM was not different between the ARAS and healthy subjects, but TCM revealed significantly lower diffusivity in ARAS.
Conclusions: Excessive renal fat may cause underestimation of renal ADC and pure-diffusivity, which may hinder detection of renal pathology. Models accounting for fat contribution may help reduce the variability of diffusivity calculated using DWI.
Keywords: Renal adiposity, Diffusion-weighted imaging, intravoxel incoherent motion, obesity.
Over the past two decades, diffusion-weighted imaging (DWI) has evolved to an important tool for studying neurological disorders (1-3), while application of this method for characterization of abdominal pathological conditions awaited improved hardware and robust pulse sequences over nearly a decade (4). In the kidney, DWI has been used to investigate chronic kidney disease (CKD) (5), renal lesions (6), and deteriorating allografts (7). Nevertheless, the contribution of tubular flow and hemodynamics to the apparent diffusion constant (ADC), the diffusion quantitative index of the single compartment mono-exponential model, complicates tissue characterization and renal DWI analysis (8). This encouraged implementation of models incorporating a larger number of compartments to differentiate pure diffusion from pseudo-diffusive components. Indeed, in the kidney the intra-voxel incoherent motion (IVIM) analytical method, which utilizes a two-compartment model associated with pure diffusion and flow, showed superiority over the mono-exponential decay model (9, 10).
However, recent studies on hepatic DWI identified fat as a potential third compartment with a significant confounding effect (11, 12), even in non-steatotic livers (13, 14) or other organs (15). Abdominal DWI is typically performed using an echo-planar imaging (EPI) readout, which uses a water-only excitation. Selected excitation or fat suppression methods prevent contribution of the fat signal associated with peaks spectrally distant from water, but cannot effectively eliminate the signal from fat components with resonance frequencies close to water proton frequency. For instance, peaks between 4.2-5.3 ppm associated with triglycerides, which account for nearly 8.7% of the total in vivo fat content, remain unsuppressed (11). Moreover, in the kidney, which is located in the vicinity of bowel, susceptibility artifacts may significantly reduce the efficacy of spectral fat suppression. Because the diffusion constant of lipid molecules is orders of magnitude smaller than that in water and remains nearly unattenuated over the conventional range of b-values, the amplitude of the fat signal, especially at high b-values, can be prominent compared to the attenuated water signal (16), and therefore has a considerable impact on DWI parameters assessment (17).
The epidemic of obesity stresses the importance of characterization of the effect of ectopic fat on DWI parameters, particularly in subjects with high body mass index (BMI). Increased renal adiposity (18, 19) may potentially interfere with interpretation of DWI in the kidney in obese subjects, but to date this effect has not been evaluated. The aim of this study was to explore the effect of renal fat accumulation and suboptimal suppression on DWI parameters. We investigated this effect using computer simulations and verified the error in a large animal model of obesity, and in healthy subjects and in the presence of renal pathological conditions in humans. We hypothesized that residual MR signal from fat causes underestimation of renal ADC and IVIM pure-diffusivity, the magnitude of which may approximate a reduction in these parameters elicited by renal pathology. Moreover, we suggest that the fat-dependency of DWI parameters may be corrected by estimating the MR signal of excessive fat using heavily diffusion-weighted images.
Assuming that an unattenuated fat signal acts as an independent compartment, we formulated our model by adding a third exponential decay term to the bi-exponential IVIM model to account for the contribution of fat:
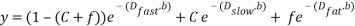 (1)
(1)
In our notation, C and 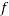 are the fractions of extravascular water and fat in the DWI signal intensity. Dfast, Dslow, andDfat are diffusion coefficients for extravascular water (pure-diffusivity), intravascular flow-dependent component (pseudo-diffusion), and fat, respectively. The product of the fat diffusion coefficient and the b-values, over the conventional range of b-values is small such that the exponential part of the third term can be approximated by one. This simplifies the last term in Equation (1) to a constant signal offset as follows:
are the fractions of extravascular water and fat in the DWI signal intensity. Dfast, Dslow, andDfat are diffusion coefficients for extravascular water (pure-diffusivity), intravascular flow-dependent component (pseudo-diffusion), and fat, respectively. The product of the fat diffusion coefficient and the b-values, over the conventional range of b-values is small such that the exponential part of the third term can be approximated by one. This simplifies the last term in Equation (1) to a constant signal offset as follows:
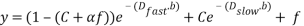 (2)
(2)
Considering that at higher b-values (~1000 s/mm2) conventionally used in DWI, the water-component of the signal intensity decays to nearly a few percent of its value at b0 (b=0 s/mm2), while the fat-related fraction (FRF), f, remains nearly unattenuated over the imaging b-value spectrum, the magnitude of FRF and its impact on calculated DWI parameters becomes significant.
I. Simulations
Simulations in this study pursued four aims. First, to show that in the absence of fat signal, the three-compartment model (TCM) reduces to IVIM. This would essentially verify that a non-zero FRF is not merely a result of overfitting the data of an intrinsically two-compartment system into a three-compartment model, and in fact represents a third independent compartment. Second, to investigate the influence of FRF, as illustrated in equation (1), on the diffusion parameters calculated using the bi-exponential IVIM model. Third, to examine the effect of signal-to-noise ratio (SNR) on the accuracy of DWI parameters assessed using IVIM and TCM, particularly since increasing the degrees of freedom in TCM per se reduces the stability of the regularization methods. Finally, to test if in the presence of fat signal the DWI parameters calculated using IVIM and TCM would be b-value dependent.
We simulated the total MR signal using the TCM, including fast and slow decays associated with intra- and extravascular fluid, as well as the FRF signal as a third compartment. Simulations were performed for diffusion parameters similar to DWI values reported for the kidney (10), over a range of FRFs (0-10%) and SNRs (2.5-50dB) (Table 1). IVIM and TCM were used to extract DWI parameters. In TCM, the total MR signal intensity for all b-values was subtracted by the signal intensity from the corresponding voxel of the high b-value (>2500 s/mm2) image, and the data were then fitted to a bi-exponential model. Table 1 shows the values used in the simulations.
To verify the b-value dependency, DWI parameters were calculated from a set of b-values with the highest value being either 600, 1000, or 2000 s/mm2.
II. Animal study
All animal procedures followed the Guideline for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996) and were approved by the Institutional Animal Care and Use Committee at Mayo Clinic.
Fourteen domestic swine in this study were fed ad lib for 16 weeks. Seven animals consumed a normal diet (Controls) and the other half (Obese) a high fat/carbohydrate diet (5B4L; Purina Test Diet, Richmond, IN) containing (in % kcal) 17% protein, 20% complex carbohydrates, 20% fructose, and 43% fat and supplemented with 2% cholesterol and 0.7% sodium cholate. We have recently shown that this diet induces obesity and adiposity (20).
Diffusion-weighted MRI scans were performed at the completion of diet. Renal volume and hemodynamics were assessed 2-3 days apart from MR scans, using multi-detector computed tomography (MDCT). Prior to each in vivo study animals were anesthetized (Telazol 5mg/kg and xylazine 2mg/kg in saline), and anesthesia maintained with intravenous ketamine (0.2 mg/kg/min) and xylazine (0.03 mg/kg/min) (for CT), or inhaled 1-2% isoflurane (for MRI) throughout the course of imaging. Blood pressure was measured using an arterial catheter during the MDCT scanning session.
Animals were injected with 10cc of heparin and euthanized with a lethal intravenous dose of sodium pentobarbital (100 mg/kg) a few days after the in vivo studies. Then the kidneys were removed and immersed in saline containing heparin. The tissue was stored at -80°C or preserved in formalin for histology.
a. Diffusion-weighted Imaging (DWI)
DWI was performed on a 3T scanner (GE Medical Systems, Milwaukee, Wisconsin) using a torso array coil. Images were collected using a single-shot echo-planar sequence with bipolar gradient. In all animals, 4-6 coronal slices in oblique planes were collected for b-values 50, 100, 200, 300, 600, 800 and 1000 s/mm2. MR parameters were set to TR/TE 1800/79ms, field of view 35cm, Bandwidth 648Hz/pixel, Number of averages 3, slice thickness 2.5mm, and matrix size 128×128. All acquisitions were performed during suspended respiration.
b. MDCT imaging
Renal hemodynamics were assessed from contrast-enhanced MDCT images, as previously detailed (21). A pigtail catheter was advanced through the left jugular vein to the superior vena cava to inject contrast media during the scan. Then animals were moved to MDCT unit (Somatom Sensation 64; Siemens Medical Solutions, Forchheim, Germany). Following localization of the kidneys, a bolus of iopamidol (0.5 ml/kg over 2s) was injected, and after a 3-second delay, 140 consecutive scans were acquired over approximately 3 minutes. After the flow scan and an additional contrast injection, a volume study was performed. Axial images were acquired at helical acquisition with thickness of 0.6mm and resolution of 512×512, and reconstructed at 5mm thickness.
c. Lipid Panel
Lipid (total cholesterol, triglyceride, high density lipid (HDL)) was measured (Roche) at the Mayo Immunochemical Core Laboratory from blood samples, and low-density lipid (LDL) was calculated.
d. Morphological Studies
Images were acquired using an ApoTome microscope (Carl ZEISS SMT, Oberkochen, Germany). Renal fibrosis was quantified by colorimetric measurements in 5µm slides stained for trichrome. Tubular dilation was measured in Periodic acid-Schiff (PAS)-stained slides counterstained with Hemotoxylin. Intracellular lipid accumulation was assessed by colorimetric measurements in Oil-Red-O stained slides from frozen tissue counterstained with Hematoxylin.
III. Human study
The study was approved by the Institutional Review Board of the Mayo Clinic, in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA) guidelines. All patients provided written informed consent before enrollment.
Fifteen patients with essential hypertension (EH) were recruited from an on-going study, to study the effect of renal fat on DWI parameters. Patients were divided in two groups based on their BMI: an obese group (n=10, BMI≥30kg/m2) and a lean group (n=5, BMI 20-25kg/m2). Additionally, diffusion parameters assessments in healthy vs. impaired (post-stenotic) kidneys, with and without fat correction, were compared in eight patients with atherosclerotic renal artery stenosis (ARAS), and five healthy controls.
a. DWI
In patients 3-8 axial images were acquired on 3T scanner (GE Medical Systems, Milwaukee, WI and Siemens Medical Systems, Erlangen, Germany) with MR parameters TR/TE, Bandwidth, Slice thickness, matrix size, and b-values were set to 2000-2400/60-94ms, 1953 Hz/pixel, 7mm, 128×128 or 160×160, and 100, 300, 600, 900 (s/mm2) in the first study with EH patients. In ARAS and Control subjects the TR/TE were 2600-4286/59-112ms. Pure-diffusivity was calculated from b-values ≥300 s/mm2 and fat-related fraction was assessed from high b-values, 2000-2500 s/mm2.
b. Clinical parameters and Lipid Panel
Clinical and laboratory parameters including age, sex, weight, BMI, blood pressure, serum creatinine, estimated glomerular filtration rate (eGFR), and lipid panel levels were evaluated at study entry by standard procedures.
IV. Data analysis
a. DWI
Pixel-by-pixel maps of quantitative indices of mono-exponential model, ADC, and bi- and tri-exponential models, IVIM and TCM parameters, respectively, were generated (Figure 1), as shown previously (22). The threshold for fast vs. slow components was set to 300s/mm2 in both animal and patient studies (23).
Large cortical regions of interest (ROIs) were drawn on b0 DWI images and transferred to the maps as detailed before (22). Mean values of ADC and IVIM parameters were calculated by averaging values in all corresponding ROIs for all slices in the subject.
b. MDCT
Using contrast-enhanced MDCT in animals, single-kidney volume, GFR, perfusion, and renal blood flow (RBF) were calculated. To calculate renal function and hemodynamics, the cortical and medullary signal attenuation vs. time curves were fitted to an extended Γ-variate model. Regional blood volumes and mean transit times were calculated to estimate cortical and medullary perfusion and blood flows (products of perfusion and the corresponding volumes). Total RBF was assessed as the sum of cortical and medullary flows. Finally, GFR was evaluated using the slope of the cortical proximal tubular curve, as previously shown (21).
Data Analysis software
All analyses were performed in MATLAB® (MathWork, Natick, MA, USA) and Analyze™ (Biomedical Imaging Resource, Mayo Clinic, MN, USA).
V. Statistical Analysis
Simulation results are shown as mean ± STD, and in vivo results as Median [First Quartile – Third Quartile]. Minimum sample size was calculated using power analysis for minimum power value of 0.8. Non-parametric Mann-Whitney was used for comparison among groups. For p values <0.05, differences were considered significant.
I. Simulations
At SNR = 50 dB, and in the absence of fat (two-compartment), TCM and IVIM estimated similar values for DWI parameters (the mean difference of pure-diffusivity calculated by the two methods was <1×10-4 mm2/s). Once FRF was included, TCM continued to yield the simulated (true) values, while ADC and IVIM underestimated the diffusion parameters. Figure 2.A shows the ADC- and IVIM-estimated diffusion parameters vs. FRF. The greatest underestimation rate occurred at low FRFs (Figure 2.B). While for SNRs <18dB TCM and IVIM both underestimated pure-diffusivity, the underestimation by TCM always exceeded that by IVIM (Figure 2.C). Moreover, selecting the upper limit of b-values of 800, 1000 and 2000 s/mm2 resulted in different IVIM estimations of pure-diffusivity, indicating IVIM b-value dependency (Figure 2.D).
II. Animal study
At 16 weeks the obese domestic swine weighed significantly more than the Controls, and lipid levels were elevated. The blood volume fraction and normalized GFR were higher in the obese (Table 2), but tubular injury scores and trichrome staining, indices of pathological conditions that might change tissue diffusion characteristics, showed no difference in the level of tubular injury, interstitial fibrosis, or glomerulosclerosis. In contrast, Oil-Red-O staining revealed macrovesicular steatosis and significantly greater intracellular lipid content in obese animals (Figure 3). DWI analysis demonstrated lower ADC (p=0.005) and pure-diffusivity (p=0.001) in the cortex of obese compared to control animals. Pure-diffusivity did not correlate with interstitial fibrosis or tubular injury score, while linear regression analysis showed a moderate but significant inverse linear and logarithmic correlations between the pure-diffusivity and the intracellular lipid content (R2=0.29, p=0.045 and R2=0.35, p=0.021, respectively).
I. Human study
In EH patients, blood pressure, hemodynamic parameters, and GFR were not different among the obese and lean groups (Table 3), but total cholesterol and triglycerides tended to be and LDL was significantly higher in Obese compared to control subjects (p=0.071, p=0.069, and p=0.026, respectively). ADC and pure diffusivity were reduced in the Obese compared to the control group (p=0.001), whereas other IVIM parameters were statistically not different between the two groups.
Finally, in ARAS patients compared to Normal controls, serum creatinine was significantly higher, and GFR was lower (Table 4). Despite a similar BMI, FRF was higher in controls compared to ARAS. Before correction for fat contribution, pure-diffusivity was similar between the two groups. After correction, pure-diffusivity significantly rose in both groups, yet was lower in ARAS vs. Control (Figure 4).
This study shows that renal adiposity confounds calculation of DWI parameters using conventional models, and may lead to underestimation of renal diffusion, even at low fractions of the fat signal. We introduced a three-compartment model that accounts for the fat contribution, and using simulations, a pig model, and in human subjects, showed that this model improves estimation of tissue integrity in obese subjects.
In the kidney, a change in tissue pure-diffusivity has been usually attributed to morphological alterations, particularly interstitial fibrosis and tubular injury (22). However, in this study, the absence of signs of tubular injury or interstitial fibrosis in obese pig kidneys, argues against their primary role in the decline in DWI parameters compared to control animals. Taken together with our simulation studies and the correlation between IVIM and renal lipid accumulation, these observations support our hypothesis that DWI parameters in the kidney are sensitive to unsuppressed fat, and that conventional models that fail to account for fat may underestimate diffusion markers.
The confounding effect of fat on hepatic DWI parameters has been suggested by several recent studies, demonstrating an inverse correlation of ADC and pure-diffusivity with fat content (12, 14), and its significance emphasized even in non-steatotic livers (13). In contrast to a previously reported study in a fat-water mixed phantom (11), our simulations and in vivo observations suggested that underestimation of renal diffusion coefficients is high at low fractions of the fat signal, and decreases until the curve nearly plateaus. Dijkstra et al showed that the correlation of pure-diffusivity and FRF is stronger using a log-linear compared to linear correlation (13), concordant with our observed correlation of this marker and Oil-Red-O. Taken together, these findings support a non-linear relationship between the FRF and pure-diffusivity (24). The importance of this observation stems from the implication that due to the non-linear behavior of pure-diffusivity, notable particularly at low FRF, the confounding effect of fat on underestimation of DWI parameters extends to early stages and to organs with traditionally limited fat deposition, like the kidney. While a reduction in pure-diffusivity has been attributed mainly to interference of free water diffusion in the presence of fat and/or enlarged hepatic cells, our simulations indicated that the inadequacy of the IVIM model alone might cause underestimations in the scale observed in vivo.
The moderate correlation of pure-diffusivity with Oil-Red-O implicates sources other than triglycerides alone in this offset signal. Large susceptibility difference in the adjacent tissues and field inhomogeneity in the abdomen are important causes of suboptimal fat suppression. Additionally, the MR signal at the boundaries of the kidneys, surrounded with perirenal fat, is prone to contamination by chemical shift-misregistered fat signal (25). Approaches developed for suppressing the fat signal in DWI included spectral fat suppression methods, which use the chemical shift difference between fat and water to selectively suppress fat, and inversion recovery-based techniques that rely on the difference in T1 relaxation times of fat and water (26, 27). However, it is important to differentiate between fat suppression for qualitative (visual) vs. quantitative purposes. Most of these methods, including hybrid methods, have remarkable performance and significantly reduce the visible fat residual signal (28). However, as we showed with simulations and in vivo, even minor residual fat signal may introduce a meaningful error to quantitative assessments of diffusion parameters. Indeed, regardless of the source of the MR fat-related component, TCM may circumvent the confounding effect.
Furthermore, our results demonstrated that while subjects with higher BMI may show greater renal fat content, this does not exclude the presence of excess renal adiposity in patients with lower BMI (<30 kg/m2) (29, 30). Pure-diffusivity and renal ADC in swine fed with high-cholesterol diet were smaller compared to pigs fed with normal chow, while lipid markers were higher in obese pigs, and histological studies detected no overt renal injury. Similarly, pure-diffusivity and ADC values were lower in Obese compared to Lean EH patients. In humans, obesity may result in ectopic renal fat deposition (31) and lipid droplet accumulation (32). Yet, although BMI was not statistically different between the groups, FRF was lower in patients with ARAS compared to Controls, possibly because the post-stenotic kidney was more fibrotic but less adipose. Hence, our results underscored that unless more comprehensive models such as TCM are used, a decline in IVIM pure-diffusivity by higher FRF might reduce the reliability of DWI and hamper distinction between healthy and diseased kidneys.
Unaccounted fat also enhances the b-value dependency of the DWI parameters, again as a consequence of inadequacy of the model. Earlier studies showed such b-value dependency of renal ADC in mono-exponential model, particularly when b-values were mostly low (<300 s/mm2), which was considerably reduced by implementing the bi-exponential IVIM with the term accounting for pseudo-diffusion [8, 10]. With fat, the b-value dependency would be more pronounced at higher b-values, which are typically used for pure-diffusivity assessment. As our simulations showed, the presence of a fat-offset signal could result in strong b-value dependency of pure-diffusivity, further highlighting the necessity of shifting to models with more compartments, like TCM.
Limitations: Due to intrinsic distortions in EPI images, which in the kidney could be enhanced by the susceptibility issues caused by bowel, co-registration with MR images with enhanced cortico-medullary differentiation is challenging. Therefore, b0 DWI images were used for cortical ROI selection, which might have introduced a selection error. Yet, this error should be small, as special care was taken to avoid medulla by confining ROIs to the outer cortex. Moreover, FRF reduction reduces the SNR. Algorithms have been suggested for optimal b-value sampling to enhance the precision in IVIM DWI parameters assessment [25]. Similar approaches may be implemented to minimize the effect of noise in the TCM. Additionally, pseudo-diffusivity estimations using Least-squares fitting are not very robust even in IVIM, and lower SNR in TCM further destabilizes estimation of this parameter. Clearly, models such as TCM with higher degrees of freedom can benefit from more powerful regression methods.
As simulations demonstrated, the higher the pure-diffusivity, the greater was its underestimation. Even though our in vivo results supported this finding, we cannot exclude a possible influence of different b-values used in human and swine studies. We also did not have biopsy samples to verify renal adiposity in patients. Alternatively, data from water-fat signal separation methods (e.g. Dixon) could potentially help to further verify sources contributing to fat-related fraction and its correlation with renal fat. Future studies with larger sample sizes and different pathological conditions are needed to validate the TCM.
In conclusion, the fat-related component of MR signal intensity remains unattenuated over a large range of b-values, and can be modeled by adding a constant coefficient to the IVIM model. Signal from components with resonance frequency close to water, imperfect fat suppression due to field inhomogeneity, and other fat-related sources, may all contribute to offset MR signal. This study further introduces a novel TCM model, which may partly account for the contribution of this excessive fat signal. The error introduced by fat may be comparable to the change in diffusion marker by pathological conditions. Considering the epidemic of obesity, this error may be increasingly encountered, and warrants implementation of appropriate correction techniques. Additional studies are needed to confirm these findings and validate the TCM approach in other renal disease models and pathological conditions.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


