Clinical Case Study on COPD
| ✅ Paper Type: Free Essay | ✅ Subject: Nursing |
| ✅ Wordcount: 3552 words | ✅ Published: 18 Sep 2017 |
Danielle McGarry
Introduction
The patient is a 76-year-old female with a history of chronic obstructive pulmonary disease (COPD). The patient is a married housewife with a 1 pack a day smoking history from age 15. The patient states that she, “only smokes a little to calm her nerves” though her husband still continues to smoke in the home. The patient reports an increasing use of oxygen in the home over the past five years and is requiring at least 2L/min continuously over the last year. She states that she always removes her nasal cannula before smoking.
Pathophysiology of COPD
Chronic obstructive pulmonary disease is “characterized by chronic inflammation of the airways, lung parenchyma (respiratory bronchioles and alveoli), and pulmonary blood vessels [with] not fully reversible airflow limitation during exhalation” (Lewis, Bucher, Heitkemper, & Harding, 2017). Previously the definition of COPD included chronic bronchitis and emphysema due to their contribution to mucus hypersecretion and destruction of alveoli respectively. However, the current definition does not include either term, instead attributing them as contributors to the disease process. (Lewis, Bucher, Heitkemper, & Harding, 2017) Chronic inflammation causes structural deviations in the lungs which leads to airflow limitation and obstruction. Air becomes trapped in the lungs which creates gas exchange abnormalities, and an increased volume of residual air, making passive expiration arduous.
As the disease process progresses, functional residual capacity is increased and air trapping causes chest hyperextension and the patient may become barrel chested and the diaphragm may flatten. Hypoxemia and hypercapnia may result from decreased gas exchange. Bullae and blebs can form, further decreasing gas exchange and putting the patient at risk of spontaneous pneumothorax. The patient needs to be monitored for signs and symptoms of pneumothorax such as decreased movement of the chest wall and hyperresonance to percussion. If this complication were to occur, it is most frequently treated by inserting a chest tube with drainage. Chronic cough can cause excess mucus production. Late in COPD, pulmonary hypertension may occur as a result of thickening of the vascular smooth muscle. Pulmonary hypertension can cause complications of COPD, such as hypertrophy of the right ventricle (cor pulmonale) and ultimately right sided heart failure. The patient should be monitored for signs and symptoms of cor pulmonale such as dyspnea upon exertion, cough, tachypnea, fatigue and an increased S2 sound. An ECG may also be used to determine right ventricular hypertrophy. When cor pulmonale occurs secondary to another disease process the treatment revolves around treating the primary disease. Oxygen therapy is also used to correct hypoxemia. (Lewis, Bucher, Heitkemper, & Harding, 2017)
Risk factors for COPD include exposure to noxious particles, especially cigarette smoke. This exposure can also originate from secondhand (passive) smoking, occupation chemicals and dusts and air pollution. Other risk factors include reoccurring respiratory tract infections in childhood, tuberculosis, asthma, aging, gender, and genetics. The genetic risk factor for COPD is a deficiency of α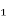 – Antitrypsin (ATT). “The main function of ATT, an α₠-protease inhibitor, is to protect normal lung tissue from attack by proteases during inflammation related to cigarette smoking and infections. Severe ATT deficiency leads to premature bullous emphysema in the lungs” (Lewis, Bucher, Heitkemper, & Harding, 2017). Low levels of ATT can be detected by a blood test. Smoking in conjunction with any of the other risk factors greatly increases the risk of COPD. (Lewis, Bucher, Heitkemper, & Harding, 2017)
– Antitrypsin (ATT). “The main function of ATT, an α₠-protease inhibitor, is to protect normal lung tissue from attack by proteases during inflammation related to cigarette smoking and infections. Severe ATT deficiency leads to premature bullous emphysema in the lungs” (Lewis, Bucher, Heitkemper, & Harding, 2017). Low levels of ATT can be detected by a blood test. Smoking in conjunction with any of the other risk factors greatly increases the risk of COPD. (Lewis, Bucher, Heitkemper, & Harding, 2017)
COPD is a progressive disease and clinical manifestations develop slowly. Early signs include chronic cough, usually with sputum production and dyspnea, especially upon exertion. Later signs of COPD are dyspnea at rest, chest breathing with use of intercostals and accessory muscles, barrel chest, weight loss, wheezing, diminished breath sounds, pursed-lipped breathing, cyanosis, and the patient sitting in a tripod position. The patient may have decreased PaOâ‚‚ and pulse oximetry reading as well as an increased PaCOâ‚‚, HCO₃⻠and hemoglobin. The patient will also have a decreased FEVâ‚ and FEVâ‚/FVC. (Lewis, Bucher, Heitkemper, & Harding, 2017) Possible symptoms include the patient complaining “of not being able to take a deep breath, heaviness in the chest, gasping, increased effort to breathe and air hunger” (Lewis, Bucher, Heitkemper, & Harding, 2017). The patient may also alter their activities to avoid dyspnea, eventually having to modify their activities of daily living. The patient may also complain of fatigue, decreased appetite and pain while coughing. (Lewis, Bucher, Heitkemper, & Harding, 2017)
The patient’s major risk factor is smoking. The patient has a 61 pack-year history (number of packs smoked per day multiplied by number of years smoked) and still currently smokes “to calm her nerves”. This risk factor includes passive smoking since the patient is exposed to environmental tobacco smoke due to her husband’s smoking in the home. The patient’s age also contributes to her risk factors since “normal aging results in a loss of elastic recoil, stiffening of the chest wall, gas exchange alteration, and decrease in exercise tolerance” (Lewis, Bucher, Heitkemper, & Harding, 2017). The patient’s gender is also a risk factor due to the belief that “women may be more susceptible to the adverse effects of smoking” (Lewis, Bucher, Heitkemper, & Harding, 2017).
The patient’s symptoms include patient report that she feels, “anxious, more fatigued than usual with a very poor appetite”, as well as increasing dyspnea described as “really bad now, I can’t breathe”. Signs observed upon assessment include the patient being pale and restless, sitting in a tripod position using pursed-lip breathing and accessory muscles during respiration. Patient is dyspneic with diminished lung sounds in the bases and diffuse rhonchi and some faint, expiratory wheezing. The patient is still within normal BMI range at 19 but has lost 4kg (8.8lbs) in three months. Her blood pressure is borderline hypotensive at 100/54. The patient is tachypneic with a respiratory rate of 34-38 and tachycardic with a pulse rate of 118-124. Her oxygen saturation is low with a SaOâ‚‚ of 88%. All of these signs and symptoms are manifestations of the patient’s inadequate oxygenation due to an exacerbation of her COPD. The patient’s lab values also reflect her disease process and are discussed under the laboratory values section.
Medical Treatments for COPD:
Home: The patient takes Spiriva Respimat (2.5 mcg/actuation), 2 inhalations once daily. Tiotropium is a long-acting anticholinergic (muscarinic antagonist), and bronchodilator. It works by inhibiting acetylcholine at receptor sites of bronchial smooth muscle, resulting in decreased cGMP and bronchodilation. This LAMA is used for long-term treatment and once-daily maintenance of bronchospasm associated with COPD. The patient uses Serevent Diskus DPI (50 mcg/inhalation), 1 puff twice a day. Salmeterol (Serevent Diskus) is a long-acting ßâ‚‚-adrenergic agonist, and bronchodilator. This LABA increases levels of cAMP, which relaxes pulmonary smooth muscle. It is used to prevent bronchospasm in COPD patients and maintains improvement in forced expiratory volume (FEV) from 3 to 12 hours. It is not used for acute exacerbations. (Lewis, Bucher, Heitkemper, & Harding, 2017) (Skidmore-Roth, 2017) Emergency Department: The patient was prescribed Solu-medrol, 50 mg IV, every 6 hours. Methylprednisolone (Solu-medrol) is a corticosteroid (glucocorticoid), an anti-inflammatory. Solu-medrol’s anti-inflammatory process works by suppressing the migration of fibroblasts and leukocytes, decreasing capillary permeability and lysosomal stabilization. It is used to decrease inflammation for management of acute exacerbation of COPD. The patient was also prescribed DuoNeb solution (500 mcg ipratropium and 2500 mcg albuterol in 3-mL) via nebulizer STAT and every 6 hours. DuoNeb is a combination of ipratropium and albuterol. Ipratropium (Atrovent) is an anticholinergic and bronchodilator. It has the same method of action as Tiotropium, inhibiting acetylcholine at receptor sites on bronchial smooth muscle, resulting in decreased cGMP and bronchodilation. Albuterol (Proventil) is a ßâ‚‚-adrenergic agonist, bronchodilator, and sympathomimetic. Albuterol has the same method of action as Salmeterol, acting on ßâ‚‚ pulmonary receptors by increasing cAMP which relaxes bronchial smooth muscle. Both of these medications are indicated for COPD, though Albuterol is short acting (SABA) and specifically targets acute bronchospasm. (Skidmore-Roth, 2017) By combining these bronchodilators, DuoNeb “improves their effect and decreases the risk of adverse effects” (Lewis, Bucher, Heitkemper, & Harding, 2017). The patient is also on supplemental oxygen via nasal cannula at 3L/min and IV fluids of normal saline at 80 mL/hr.
Nursing Treatments for COPD:
Administer medications (Solu-medrol and DuoNeb) to promote airway patency and gas exchange. Auscultate breath sounds, noting areas of decreased or absent breath sounds and adventitious sounds to obtain ongoing data on patient’s response to therapy. Monitor respiratory and oxygenation status to assess need for intervention and give supplemental oxygen therapy as ordered to increase PaOâ‚‚ and improve Oâ‚‚ saturation levels. Monitor the effectiveness of this therapy via ABGs and pulse oximetry to evaluate the patient response to therapy. If increased Oâ‚‚ therapy of more than 35-50% is ordered, use a humidification or nebulization device to prevent drying secretions and mucous membranes. Teach and encourage pursed-lip breathing to prolong exhalation thereby preventing air trapping and to slow the respiratory rate. Encourage slow, deep breathing; turning; and coughing to promote effective breathing techniques and secretion mobilization. If patient is able to expectorate sputum, assess and monitor sputum volume and purulence to detect possible infection. If the patient is having trouble expectorating, teach and promote airway clearance techniques such as huff coughing to loosen secretions and promote expectoration. Position the patient to minimize respiratory efforts: elevate the head of the bed, have patient sit in high fowlers and provide an overbed table for the patient to lean on, in order to promote chest expansion and effective breathing and save energy. (Lewis, Bucher, Heitkemper, & Harding, 2017) “Assess the patient’s ABG’s for any movement toward respiratory acidosis and further hypoxemia, indicating respiratory failure” (Lewis, Bucher, Heitkemper, & Harding, 2017) to prevent respiratory failure and to detect a need for assistance. “Assess the patient’s medical history for the stage of COPD as noted by the level of FEVâ‚” (Lewis, Bucher, Heitkemper, & Harding, 2017) in order to monitor progression of the disease. (Lewis, Bucher, Heitkemper, & Harding, 2017)
Once the exacerbation has subsided, nursing interventions would focus on patient teaching. The patient has recently lost weight, so nutritional therapy is important. Provide the patient with printed information on nutrient dense foods to promote adequate nutrition. Recommend a “diet high in calories and protein, moderate in carbohydrate, and moderate to high in fat” (Lewis, Bucher, Heitkemper, & Harding, 2017). Suggest the patient rest at least 30 minutes before eating and break up meals into five or six small meals a day to conserve energy. Assess the patient’s ability to perform ADL’s and offer alterations to activities to prevent exacerbations. Assess the patient’s ability and technique when using MDIs and DPIs via patient demonstration in order to confirm proper use of medications and alter or simplify medication regime if necessary. Assess for co-morbidities such as osteoporosis, cardiovascular disease or psychologic problems to ensure patient comprehension and safe management of disease. Use the “5 A’s” (ask, advise, assess, assist and arrange) to assess patient willingness to quit smoking. If patient appears resistant to total cessation of smoking, use the “5 R’s” (relevance, risks, rewards, roadblocks, and repetition) to motivate the patient to quit smoking. Counseling the patient and her husband in smoking cessation is vital for this patient in order to decrease exacerbations and slow the progression of the disease. Recommend the patient to a pulmonary rehabilitation clinic and/or a support group to promote smoking cessation. (Lewis, Bucher, Heitkemper, & Harding, 2017)
Evidence-based recommendations for the disease are similar to the actual treatment plan which was garnered from textbook information as well as student education. Medication therapy did not significantly differ in that short-acting bronchodilators were recommended as first line therapy to treat breathlessness though antibiotics were also recommended “in exacerbations that are characterized by an increase in sputum purulence” (Booker, 2005). This treatment is appropriate due to the rationale; the antibiotic is indicated if the exacerbation is in response to infection. This intervention is applied after a sputum culture to identify the cause of infection. If this patient was producing sputum it would need to be collected and cultured and antibiotics would be prescribed, if appropriate. Some nursing interventions also focus on self-management of COPD exacerbations and “are aimed at helping patients to recognize and respond promptly and appropriately to an exacerbation” (Booker, 2005), though it was also stated that the effectiveness of self-management has not been proven (Booker, 2005). Oxygen therapy is also recognized as a primary treatment. Another treatment suggested that was not originally in the treatment plan is medication for the patient’s anxiety related to their increased work of breathing. (Bailey, Colella, & Mossey, 2004) However, this study also indicated that the nurses believed anxiety to be the cause of the breathlessness, not that difficulty breathing caused anxiety. This study also found that “a pattern of particular patient and nursing care behaviors were identified in the nurse caregiver stories and constituted what we have designated as a COPD-illness template” (Bailey, Colella, & Mossey, 2004). This indicated that most COPD patients are treated very similarly regardless of the nurse delivering the care. (Bailey, Colella, & Mossey, 2004) Another study suggested an intervention called COPD-Guidance, Research on an Illness Perception (COPD-GRIP), a questionnaire, so that the nurse may assess the patient’s illness perception. This intervention was developed to meet the health needs of COPD patients “such as the need of a better understanding of the sustained symptom burden, physical limitations, and psychological impact of COPD” (Weldam, Lammers, Zwakman, & Schuurmans, 2017). This intervention is aimed at treating the patient as a whole, including improving their health-related quality of life. The results indicated “the nurses described the intervention as a useful, structured and individualized tool to guide COPD patients in living with the consequences of COPD” (Weldam, Lammers, Zwakman, & Schuurmans, 2017).
Laboratory Values and Diagnostic Testing:
Lab values for this patient are located in table 1 in the appendix.
The patient’s white blood cell count is elevated which is indicative of an infection. “The primary causes of [COPD] exacerbations are bacterial or viral infections” (Lewis, Bucher, Heitkemper, & Harding, 2017). If the patient is producing sputum, a sample should be collected and cultured. This will reveal if the cause of the exacerbation is an infection and whether it is bacterial or viral. The result of the culture will also determine if antibiotics are necessary and what type of antibiotic is appropriate for treatment. The patient’s hemoglobin level is also increased. This is most likely due to the chronic, inadequate oxygenation the patient faces due to her disease process. Her blood oxygen level is chronically low which is compensated by her body increasing the amount of red blood cells in an attempt to carry more oxygen. All of the ABG abnormalities are related to each other. The patient’s pH is low at 7.25 which indicates acidosis. Acidosis is also indicated by the patient’s increased carbon dioxide. Respiratory acidosis is caused by a build-up of COâ‚‚, resulting in increased carbonic acid in the blood. The increased carbonic acid dissociates, resulting in increased H⺠which is the cause of the patient’s decreased pH. Renal compensation of respiratory acidosis manifests in increased levels of HCO₃⻠and increased levels of H⺠in urine. The patient’s HCO₃⻠should also be monitored to observe renal compensation and to monitor for decompensation and metabolic alkalosis. (Lewis, Bucher, Heitkemper, & Harding, 2017) The patient also has a low PaOâ‚‚ and low oxygen saturation. These low levels are due to the air trapped in the patient’s lungs which decreases gas exchange as well as the patient’s dyspnea. The patient’s FEVâ‚ was 40% of expected value. This diagnostic value determines the medication treatment. Combination therapy with long-acting inhaled anticholinergics or corticosteroids and ßâ‚‚-agonist are indicated for any patient with a FEVâ‚ less than 60% of the expected value. (Lewis, Bucher, Heitkemper, & Harding, 2017) DuoNeb and Solu-medrol, the medications ordered in the emergency room, were ordered to decrease inflammation and dilate the bronchioles, therefore increase the amount of available oxygen for the patient. This will also increase the patient’s FEVâ‚ level. The patient also uses supplemental oxygen via nasal cannula to increase her PaOâ‚‚ and oxygen saturation levels. Additional nursing interventions to increase these abnormal lab values are patient positioning and pursed-lip breathing. These interventions and additional interventions are discussed during nursing and medical treatments for the disease process. A chest x-ray showed an increased AP diameter, flattening of the diaphragm, and decreased lung markings. These are all physical manifestations of COPD as aforementioned in the pathophysiology of COPD. Decreased lung markings are due to the enlarged air spaces. There is minimal consolidation in the right upper lung. The patient is scheduled for follow up radiography in 24 hours. The patient should have continued radiography in order to monitor for bullae or blebs. Radiography is also used to monitor for complications. Large pulmonary vessels could indicate cor pulmonale. If cor pulmonale is suspected an ECG may also be used to detect right-sided enlargement and BNP levels can be monitored since increased BNP levels indicate increased stretch. The lab values should also continue to be monitored. The patient should also use spirometry to measure their FEVâ‚/FVC ratio to monitor the effects of the medications. (Lewis, Bucher, Heitkemper, & Harding, 2017)
Medications:
The patient’s medications, both those used at home and those ordered in the emergency department, are discussed in the medical treatment section. The medical treatment section includes the medication, it’s classification, method of action, dose and frequency, as well as the reason the patient is taking the medication. Dose range, route, adverse effects, patient education, implementation, and assessment for these medications are located in table 2 in the appendix.
References
Bailey, P., Colella, T., & Mossey, S. (2004). COPD-intuition or template: nurses’ stories of acute exacerbations of chronic obstructive pulmonary disease. Journal of Clinical Nursing , 756- 764.
Booker, R. (2005, 09 09). COPD– non-pharmacological approaches, exacerbations, oxygen therapy and care delivery. Practice Nurse, 30(4), 59-68. Retrieved March 21, 2017
Lewis, S., Bucher, L., Heitkemper, M., & Harding, M. (2017). Medical-Surgical Nursing Assessment and Management of Clinical Problems. St. Louis: Elsevier.
Skidmore-Roth, L. (2017). Mosby’s 2017 Nursing Drug Reference . St. Louis: Elsevier.
Weldam, S., Lammers, J.-W., Zwakman, M., & Schuurmans, M. (2017). Nurses’ perspectives of a new individualized nursing care intervention for COPD patients in primary care settings: A mixed method study. Applied Nursing Research, 33, 85-92.
Appendix:
|
Table 1 Laboratory Values |
|||
|
Laboratory Test |
Laboratory Value |
Reference Range Adults |
Indication |
|
White blood cell count (µl) |
11.8 |
4.8-10.8 x 103/ µl |
High |
|
Hemoglobin (g/dL) |
19 g/dL |
12-15 g/dL (women) |
High |
|
ABG: pH |
7.25 |
7.35-7.45 |
Low/critical |
|
ABG: PaCOâ‚‚ (mm Hg) |
60 |
35-45 mm Hg |
High |
|
ABG: PaOâ‚‚ (mm Hg) |
59 |
75-100 mm Hg |
Low |
|
ABG: Oxygen saturation (POX) |
89% |
96-100% |
Low |
|
Peak Flow (FEVâ‚) |
40% of expected value |
>80% of expected value |
Low |
|
Table 2 Medications |
|||||
|
Tiotropium (Spiriva Respimat) |
|||||
|
Dose Range Adult inhale content of 1 cap/day (18 mcg) using HandiHaler inhalation device or 2 INH (spray) 2.5 mcg each daily |
Route Inhalation |
Adverse Effects CNS depression, paresthesia, chest pain, increased heart rate, dry mouth, blurred vision, glaucoma, vomiting, abdominal pain, constipation, dyspepsia, urinary difficulty, rash, angioedema, cough, upper RTI, candidiasis, flulike syndrome. |
Patient Education This medication is contraindicated if the patient also takes atropine and is pregnancy category C. This medication is not for immediate relief of breathing problems. Rinse mouth after use. Avoid getting powder in eyes. Immediately report blurred vision, eye pain or halos. Store at room temperature. |
Implementation Breath in slowly and deeply, hold breath and remove mouthpiece, then resume normal breathing. |
Assessment Respiratory status |
|
Salmeterol (Serevent Diskus) |
|||||
|
Dose Range Adult inhale 50 mcg (1 inhalation as dry powder) every 12 hours |
Route Inhalation powder |
Adverse Effects Tremors, anxiety, insomnia, headache, dizziness, fever, tachycardia, palpations, hyper/hypotension, angina, dysrhythmias, dry nose, heartburn, N&V, abdominal pain, muscle cramps, bronchospasm, cough. |
Patient Teaching This medication has drug-drug interactions with CPY3A4 inhibitors, aerosol bronchodilators, tricylics, MAOI’s and ß-blockers. This drug can cause paradoxical bronchospasm with dyspnea, wheezing, and chest tightness. Avoid OTC medications. Avoid smoking. Immediately report dyspnea after use if ≥ 1 canister is used in 2 months time. Notify provider if >4 inhalations are needed. Not for treatment of acute exacerbations. |
Implementation When 2 puffs are indicated, wait 1 minute between doses. Do not use a spacer. |
Assessment Respiratory status & paradoxical bronchospasm |
|
Methylprednisolone (Solu-medrol) |
|||||
|
Dose Range Adult PO/IV 40-80 mg/day in 1-2 divided doses |
Route PO or IV |
Adverse Effects Depression, flushing, sweating, headache, mood changes, circulatory collapse, thrombophlebitis, embolism, tachycardia, hyperglycemia, adrenal suppression/insufficiency, blurred vision, diarrhea, nausea, GI hemorrhage, peptic ulcer, pancreatitis, thrombocytopenia, poor would healing, osteoporosis, poor growth in children, candidiasis, dysphonia |
Patient Teaching Do not use with grapefruit juice, long-term po glucocorticoids must be given increased doses during times of increased physiologic stress, do not discontinue abruptly, increase intake of potassium, calcium & protein, carry emergency ID, take PO with food, avoid OTC products. Drug-drug interactions with oral contraceptives, estrogens, diuretics, St. John’s wart |
Implementation Do not give intrathecally. Use only Solu-Medrol IV, never use methylPREDNISolone acetate suspension IV. Titrated dose, use lowest effective dose. |
Assessment Potassium depletion, cardiac symptoms, mental status, BP every 4 hours, I&O ratio, adrenal insufficiency& infection |
|
Ipratropium + Albuterol (DuoNeb) |
|||||
|
Dose Range (0.5 mg- 3mg) 2 INH q4-6hrs PRN |
Route Inhalation |
Adverse Effects Stevens-Johnson Syndrome, hepatotoxicity, severe and fatal immune-mediated endocrinopathies and enterocolitis, hepatitis, pancreatitis, n/v/d, urticaria, cough, dyspnea, toxic epidermal necrolysis, paradoxical bronchospasm, tremors, anxiety, insomnia |
Patient Teaching Immediately report allergic reaction, rash, severe abdominal pain, yellowing of skin and/or eyes, tingling in the extremities or change in bowel habits. Use as prescribed. Drug-drug interactions. Avoid getting aerosol in eyes. Store at room temperature in a light resistant container. |
Implementation Give INH at least 1 minute apart. |
Assessment Respiratory function and paradoxical bronchospasm. Patient’s ability to self-medicate. For evidence of allergic reactions or palpations. |
(Skidmore-Roth, 2017)
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


