Energy Efficiency of a PRO Process
| ✅ Paper Type: Free Essay | ✅ Subject: Engineering |
| ✅ Wordcount: 3031 words | ✅ Published: 30 Aug 2017 |
- Introduction
The global energy demand is expeditiously increasing due to rapidly expanding population and their improved living standard. Although fossil fuels are mostly contributing to fulfilling this demand, the consumption has already exceeded the capacity of sustainable energy production (Efraty, 2013)(Yip et al., 2011). It is often claimed that we have enough reserves of coal, gas, and oil while the real scenario is different. Environment scientists reported that energy reserves are decreasing with time, which would be diminished within few decades (Figure 1). The lifetime of these reserves would be extended slightly if new reservoirs can be identified. Discovering new wells is becoming harder day-by-day and if it is discovered, the amounts of fuels would be significantly lower than the ones that have been found in the past1.
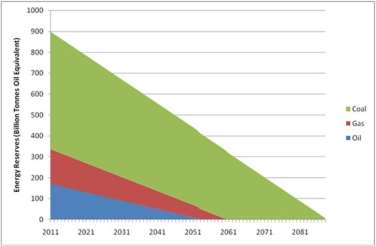
Figure 1: The trends of global fossil fuels reserves[1]
The rising energy demand and limited reserves of fossil fuels have motivated to researchers for exploring alternatives sources of renewable energy. Researchers have already discovered various sources of energy while wind, solar, tidal and biomass have been used for sustainable energy production (Straub, Deshmukh, & Elimelech, 2015). However, expensive equipment and high installation cost coupled with the uneven distribution of energy throughout the year have prevented them from being used widely (Sharif, Merdaw, Aryafar, & Nicoll, 2014). Recently, a newly emerging source of clean energy called ‘Osmotic power’ has attracted much attention to the researcher, which derived from salinity gradients found worldwide where two sources of water with different salinities are available next to each other (Y. C. Kim & Elimelech, 2013). The availability and predictability of osmotic power are much greater than the intermittent renewables like wind and solar.
Salinity gradient is the difference in salt concentration between two solutions. The enormous amount of energy released from the mixing of two solutions of different salinities and this amount rises for higher concentration difference between the solutions. Small-scale investigations have been done for the mixing of freshwater and seawater, which reported that 2.6 MW energy produced for a flow of 1m3/s freshwater when mixed with seawater (Veerman, Saakes, Metz, & Harmsen, 2009). Several technologies are being used to harvest osmotic power such as reverse electrodialysis (RED) (Achilli & Childress, 2010) (Yip & Elimelech, 2012), pressure retarded osmosis (PRO) (Altaee & Sharif, 2015)(Thorsen & Holt, 2009)(Norman & S., 2016), capacitive mixing (CAPMIX) (Reuters News Agency, n.d.), and hydrogel mixing (J. Kim, Jeong, Park, Shon, & Kim, 2015). Among the technologies, RED and PRO are more advanced and demonstrated at pilot scale and both converts chemical potential to useful work by the controlled mixing of two solutions of different salt concentration (Achilli & Childress, 2010)(Yip & Elimelech, 2014).
RED is a membrane-based technology, which is driven by the Nernst potential, a manifestation of chemical potential difference. It uses a stack of altering ion exchange membranes that selectively allows ion permeation across the membranes. The net ion flux across the membranes is converted directly to electric current (Norman & S., 2016)(Pattle, 1954). The process is very efficient for power generation but economically inefficient. The cost prices of available RED membrane is out of range, and recent investigations have showed that the price has to be reduced a hundred times to make the technology affordable (Post et al., 2010). The development of such type of membranes is very time consuming and difficult to achieve (Turek & Bandura, 2007). Also, The operations of the RED process is complex and highly sensitive to the process parameters, which requires elaborate control system (Altaee & Sharif, 2015).
Alike reverse electrodialysis, PRO is also a membrane-based technology, but the difference is, PRO uses a single salt-rejecting semipermeable membrane instead of a stack of ion-exchange membranes. It utilizes the salinity gradient as osmotic power difference to drive the water permeation across the membrane from low salinity ‘feed’ solution to high salinity ‘draw’ solution. The expanding volume of draw solution flows through a hydro-turbine that generates useful mechanical and electrical works [18][19]. The design and operations of PRO are much simpler, and it does not depend too much on operational parameters except operating pressure of membrane at draw solution side. The recent analysis shows that PRO can achieve both greater efficiencies and power densities than RED and other existing technologies [14].
Most of the PRO studies have been focused on the mixing of seawater and freshwater, but this mixing scheme has been found to be unfeasible due to the lower power densities. Researchers agree that more study is necessary to assess the feasibility of processes based on streams of higher salinity. One of such processes is the energy recovery from desalination units by taking advantages of the mixing of discharged brine and seawater. Another process is the mixing of seawater with high salinity produced water from oil and natural gas exploration. However, the main problems of these process are concentraion polarization and salt leakage, which limit the PRO performance by reducing the driving force across the membrane. Before investigations to establish a viable PRO process for the large-scale operation, have focused on developing high-performance membrane and setting up suitable conditions to maximize the energy yields.
Several thermodynamic properties are necessary to set up appropriate conditions to assess the performance of PRO process. The first of them is the Gibbs free energy of mixing because it provides the upper limit to the shaft power that is possible to recover from a mixing process, which occurs at constant temperature and pressure. Another property is osmotic pressure, which in necessary to establish operating pressure at different parts of the plant. Entropies and enthalpies are needed to evaluate the mechanical power of the rotary equipment involved. This work demonstrates a thermodynamic model to evaluate all of them in order to maximize the power recovery from PRO process. The Q-electrolattice equation of (EOS), which extends a lattice-based fluid model for electrolyte solutions, is adopted. The model also includes recently developed equations for PRO that considers concentration polarization; reverse salt permeability, and membrane fouling to predict water and salt flux across the membrane.
In addition, most PRO models are based on solutions of Na+ and Cl– ions only, whereas, in practice, saline water contains other ions in addition to these two. This work reports simulations of PRO processes that consider the presence of multiple ions in solutions (Na+, K+, Mg2+, Ca2+, Cl- and SO42-). The existing model mostly uses different platforms to calculate osmotic power, power density, and flux across the membrane (e.g. OLI-software is used to calculate osmotic power and another program for flux and power density), that increase the possibility of getting erroneous value because all these are inter-dependent. On the other hand, this model constantly and accurately determines all of them by a single program.
Initial investigations have been done for freshwater+sewater and seawater+brine systems with single-stage PRO configuration. The predicted osmotic pressure, water flux across the membrane and recoveries of mechanical power are in very good agreement with experimental literature data. This set of results suggests that the Q-electrolattice EOS is a suitable model for the calculation of thermodynamic properties needed to assess the performance of PRO plants. Now, it is planning this model for very high salinity solutions with multiple stage configurations. A techno-economic analysis will be done for the feasibility study of PRO process implementing at industrial scale.
Aim and Objectives:
The aim of this work is to develop a thermoynamic model based on Q-electrolattice equation of state for PRO process, and implement it to predict different thermodynamic properties in order to caltulate water and salt flux across the membrane and power densities. The various objectives associated with this aim are delineated below:
- Implement Q-electrolattice equation of state for the solutions of multiple salts to calculate osmotic power and verify the results with literature experimental data.
- Implement recently developed mass and salt flux equations, which considered concentration polarization, reverse salt flux and fouling of membrane.
- Implement basic thermodynamic relations for PRO units to determine entropies and enethalpies accurately.
- Develop the model for freshwater-seawater system with single stage configuration and extended it for higher salinity system with multiple stage configuration.
- Implement the cost equations to determine the capital cost for installation of the PRO units.
- Literature Review
- Q-elctrolattice equation of state
The elctrolattice equation of state (EOS) was developed using the same methodology presented by Myers et al. (Myers, Sandler, & Wood, 2002), based on the Helmholtz energy approach. The residual Helmholtz energy at a given temperature and volume is calculated by the addition various contributions along a hypothetical path. These contributions consist of ion-solvent and solvent-solvent interaction over the short range, solvation effects, and ion-ion interactions over the long range.
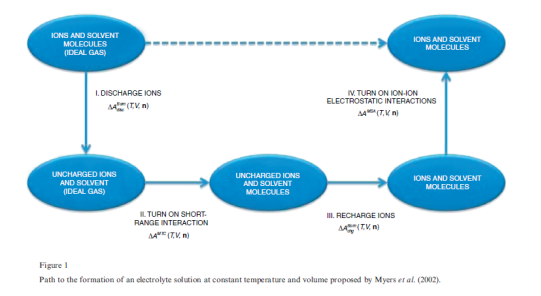
The total process is divided into four steps along a thermodynamic path( a. Zuber et al., 2013):
Step-1: It is assumed that a reference mixture consisting of charged ions and molecules is in a hypothetical ideal gas state at temperature T and volume V. In the first step, the charges on all ions are removed. The change in Helmholtz energy is accounted by the Born equation for ions in a vacuum, 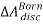
Step-2: The short-range attractive dispersion and repulsive forces due to excluded volume are turned on. Also, self-association of solvent molecules can occur. The MTC EOS is used to calculate the change in Helmholtz energy for this step,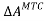 .
.
Step-3: The ions are recharged. The change in Helmholtz energy is accounted for by the Born equation for ions in a dielectric solvent, 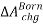
Step-4: The long-range interactions among the ions in solution are taken into account using the Mean Spherical Approximation (MSA), and the corresponding change in the molar Helmholtz free energy is denoted by 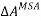 .
.
The residual Helmholtz energy for forming an electrolyte solution is thus given by:
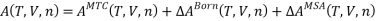
wherein
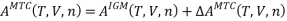
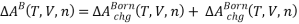
So,
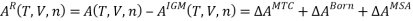
To model electrostatic interactions, a single salt electrolyte solution is divided into five regions: three for solvent (D, α, and β), one for cation (C) and one for anion (A).
To determine the MTC Helmholtz energy change, the model uses seven parameters to represent pure solvents. The model assumes that the region-region interaction (except for α-β) are dispersion interactions, which are temperature dependent. In addition, it also assumed that the short-range interactions between the α and β region are zero. This is summarized below:
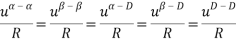
In addition, hydrogen bonding interactions are taken to be temperature independent.
It is assumed that the interaction between the solvent and each charged species is equal; short-range interaction between opposite ions and same charge are neglected altogether. This is summarized below:
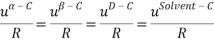
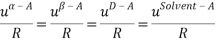
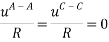
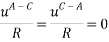
The Q-electrolattice equation of state is an extended version of the EOS in which an explicit MSA term is used which allows for unequal ionic diameters (which are ultimately regressed using experimental data).
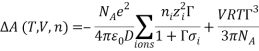
- PRO principles
- Basic Theory
Reference:
Achilli, A., & Childress, A. E. (2010). Pressure retarded osmosis: From the vision of Sidney Loeb to the first prototype installation – Review. Desalination, 261(3), 205-211. https://doi.org/10.1016/j.desal.2010.06.017
Altaee, A., & Sharif, A. (2015). Pressure retarded osmosis: advancement in the process applications for power generation and desalination. In Desalination (Vol. 356, pp. 31-46). Elsevier B.V. https://doi.org/10.1016/j.desal.2014.09.028
Efraty, A. (2013). Pressure retarded osmosis in closed circuit: a new technology for clean power generation without need of energy recovery. Desalination and Water Treatment, 51(40-42), 7420-7430. https://doi.org/10.1080/19443994.2013.793499
Kim, J., Jeong, K., Park, M. J., Shon, H. K., & Kim, J. H. (2015). Recent advances in osmotic energy generation via pressure-retarded osmosis (PRO): A review. Energies, 8(10), 11821-11845. https://doi.org/10.3390/en81011821
Kim, Y. C., & Elimelech, M. (2013). Potential of osmotic power generation by pressure retarded osmosis using seawater as feed solution: Analysis and experiments. Journal of Membrane Science, 429, 330-337. https://doi.org/10.1016/j.memsci.2012.11.039
Myers, J. a., Sandler, S. I., & Wood, R. H. (2002). An Equation of State for Electrolyte Solutions Covering Wide Ranges of Temperature, Pressure, and Composition. Industrial & Engineering Chemistry Research, 41(13), 3282-3297. https://doi.org/10.1021/ie011016g
Norman, S. L., & S., R. (2016). Osmotic Power Plants Author ( s ): Sidney Loeb and Richard S . Norman. Science, 189(4203), 654-655.
Pattle, R. E. (1954). Production of Electric Power by mixing Fresh and Salt Water in the Hydroelectric Pile. Nature.
Post, J. W., Goeting, C. H., Valk, J., Goinga, S., Veerman, J., Hamelers, H. V. M., & Hack, P. J. F. M. (2010). Towards implementation of reverse electrodialysis for power generation from salinity gradients. Desalination and Water Treatment, 16(1-3), 182-193. https://doi.org/10.5004/dwt.2010.1093
Reuters News Agency. (n.d.). Norway Opens World’s First Osmotic Power Plant. Retrieved January 17, 2013, from http://www.reuters.com/article/2009/11/24/us-nor way-osmotic-idUSTRE5A-N20Q20091124
Sharif, A., Merdaw, A., Aryafar, M., & Nicoll, P. (2014). Theoretical and Experimental Investigations of the Potential of Osmotic Energy for Power Production. In Membranes (Vol. 4, pp. 447-468). https://doi.org/10.3390/membranes4030447
Straub, A. P., Deshmukh, A., & Elimelech, M. (2015). Pressure-retarded osmosis for power generation from salinity gradients: is it viable? Energy Environ. Sci. https://doi.org/10.1039/C5EE02985F
Thorsen, T., & Holt, T. (2009). The potential for power production from salinity gradients by pressure retarded osmosis, 335, 103-110. https://doi.org/10.1016/j.memsci.2009.03.003
Turek, M., & Bandura, B. (2007). Renewable energy by reverse electrodialysis. Desalination, 205(1-3), 67-74. https://doi.org/10.1016/j.desal.2006.04.041
Veerman, J., Saakes, M., Metz, S. J., & Harmsen, G. J. (2009). Reverse electrodialysis: Performance of a stack with 50 cells on the mixing of sea and river water. Journal of Membrane Science, 327(1-2), 136-144. https://doi.org/10.1016/j.memsci.2008.11.015
Yip, N. Y., & Elimelech, M. (2012). Thermodynamic and energy efficiency analysis of power generation from natural salinity gradients by pressure retarded osmosis. Environmental Science and Technology, 46(9), 5230-5239. https://doi.org/10.1021/es300060m
Yip, N. Y., & Elimelech, M. (2014). Comparison of Energy Efficiency and Power Density in Pressure Retarded Osmosis and Reverse Electrodialysis (7th Editio).
Yip, N. Y., Tiraferri, A., Phillip, W. A., Schiffman, J. D., Hoover, L. A., Kim, Y. C., & Elimelech, M. (2011). Thin-film composite pressure retarded osmosis membranes for sustainable power generation from salinity gradients{_}. Environmental Science and Technology, 45(10), 4360-4369. https://doi.org/10.1021/es104325z
Zuber, A., Figueiredo, R., & Castier, M. (2014). Fluid Phase Equilibria Thermodynamic properties of aqueous solutions of single and multiple salts using the Q-electrolattice equation of state. Fluid Phase Equilibria, 362, 268-280.
Zuber, a., Checoni, R. F., Mathew, R., Santos, J. P. L., Tavares, F. W., & Castier, M. (2013). Thermodynamic Properties of 1:1 Salt Aqueous Solutions with the Electrolattice Equation of State. Oil & Gas Science and Technology – Revue d’IFP Energies Nouvelles, 68(2), 255-270. https://doi.org/10.2516/ogst/2012088
This work focuses on developing a thermodynamic model to analyse the energy efficiency of a PRO process in order to maximize the power recovery. It uses Q-electrolattice equation of state (developed for mixtures with mixed electrolytes) that can accurately determine various thermodynamics properties such as vapor pressure, osmotic coefficient, osmotic pressure, entropy and enthalpy at different conditions of concentration temperature and pressure (A. Zuber, Figueiredo, & Castier, 2014). The model is implemented to XSEOS excel tool to calculate these thermodynamic properties. Moreover, it does not have any limitations to calculate osmotic pressure and other properties for very high concentraion solution containing multiple salts at extreme high temperation and pressure conditions.
Achilli, A., & Childress, A. E. (2010). Pressure retarded osmosis: From the vision of Sidney Loeb to the first prototype installation – Review. Desalination, 261(3), 205-211. https://doi.org/10.1016/j.desal.2010.06.017
Altaee, A., & Sharif, A. (2015). Pressure retarded osmosis: advancement in the process applications for power generation and desalination. In Desalination (Vol. 356, pp. 31-46). Elsevier B.V. https://doi.org/10.1016/j.desal.2014.09.028
Efraty, A. (2013). Pressure retarded osmosis in closed circuit: a new technology for clean power generation without need of energy recovery. Desalination and Water Treatment, 51(40-42), 7420-7430. https://doi.org/10.1080/19443994.2013.793499
Kim, J., Jeong, K., Park, M. J., Shon, H. K., & Kim, J. H. (2015). Recent advances in osmotic energy generation via pressure-retarded osmosis (PRO): A review. Energies, 8(10), 11821-11845. https://doi.org/10.3390/en81011821
Kim, Y. C., & Elimelech, M. (2013). Potential of osmotic power generation by pressure retarded osmosis using seawater as feed solution: Analysis and experiments. Journal of Membrane Science, 429, 330-337. https://doi.org/10.1016/j.memsci.2012.11.039
Myers, J. a., Sandler, S. I., & Wood, R. H. (2002). An Equation of State for Electrolyte Solutions Covering Wide Ranges of Temperature, Pressure, and Composition. Industrial & Engineering Chemistry Research, 41(13), 3282-3297. https://doi.org/10.1021/ie011016g
Norman, S. L., & S., R. (2016). Osmotic Power Plants Author ( s ): Sidney Loeb and Richard S . Norman. Science, 189(4203), 654-655.
Pattle, R. E. (1954). Production of Electric Power by mixing Fresh and Salt Water in the Hydroelectric Pile. Nature.
Post, J. W., Goeting, C. H., Valk, J., Goinga, S., Veerman, J., Hamelers, H. V. M., & Hack, P. J. F. M. (2010). Towards implementation of reverse electrodialysis for power generation from salinity gradients. Desalination and Water Treatment, 16(1-3), 182-193. https://doi.org/10.5004/dwt.2010.1093
Reuters News Agency. (n.d.). Norway Opens World’s First Osmotic Power Plant. Retrieved January 17, 2013, from http://www.reuters.com/article/2009/11/24/us-nor way-osmotic-idUSTRE5A-N20Q20091124
Sharif, A., Merdaw, A., Aryafar, M., & Nicoll, P. (2014). Theoretical and Experimental Investigations of the Potential of Osmotic Energy for Power Production. In Membranes (Vol. 4, pp. 447-468). https://doi.org/10.3390/membranes4030447
Straub, A. P., Deshmukh, A., & Elimelech, M. (2015). Pressure-retarded osmosis for power generation from salinity gradients: is it viable? Energy Environ. Sci. https://doi.org/10.1039/C5EE02985F
Thorsen, T., & Holt, T. (2009). The potential for power production from salinity gradients by pressure retarded osmosis, 335, 103-110. https://doi.org/10.1016/j.memsci.2009.03.003
Turek, M., & Bandura, B. (2007). Renewable energy by reverse electrodialysis. Desalination, 205(1-3), 67-74. https://doi.org/10.1016/j.desal.2006.04.041
Veerman, J., Saakes, M., Metz, S. J., & Harmsen, G. J. (2009). Reverse electrodialysis: Performance of a stack with 50 cells on the mixing of sea and river water. Journal of Membrane Science, 327(1-2), 136-144. https://doi.org/10.1016/j.memsci.2008.11.015
Yip, N. Y., & Elimelech, M. (2012). Thermodynamic and energy efficiency analysis of power generation from natural salinity gradients by pressure retarded osmosis. Environmental Science and Technology, 46(9), 5230-5239. https://doi.org/10.1021/es300060m
Yip, N. Y., & Elimelech, M. (2014). Comparison of Energy Efficiency and Power Density in Pressure Retarded Osmosis and Reverse Electrodialysis (7th Editio).
Yip, N. Y., Tiraferri, A., Phillip, W. A., Schiffman, J. D., Hoover, L. A., Kim, Y. C., & Elimelech, M. (2011). Thin-film composite pressure retarded osmosis membranes for sustainable power generation from salinity gradients{_}. Environmental Science and Technology, 45(10), 4360-4369. https://doi.org/10.1021/es104325z
Zuber, A., Figueiredo, R., & Castier, M. (2014). Fluid Phase Equilibria Thermodynamic properties of aqueous solutions of single and multiple salts using the Q-electrolattice equation of state. Fluid Phase Equilibria, 362, 268-280.
Zuber, a., Checoni, R. F., Mathew, R., Santos, J. P. L., Tavares, F. W., & Castier, M. (2013). Thermodynamic Properties of 1:1 Salt Aqueous Solutions with the Electrolattice Equation of State. Oil & Gas Science and Technology – Revue d’IFP Energies Nouvelles, 68(2), 255-270. https://doi.org/10.2516/ogst/2012088
[1] All fossil fuel reserve and consumption data from CIA World Factbook
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


