Development of Organic Redox Flow Batteries for Renewable Energy
| ✅ Paper Type: Free Essay | ✅ Subject: Engineering |
| ✅ Wordcount: 2794 words | ✅ Published: 18 May 2020 |
Development of Organic Redox Flow Batteries for applications in storage of Renewable Energy
Executive Summary
Fossil fuels are still the primary source of energy in current time, but due to increasing concern towards the carbon emission from these sources and developed nations poised to prevent further climate change, the world has started shifting more towards use of renewable sources of energy. Availability of unlimited amounts of solar and wind power is the major propagator of this shift. However, we cannot control the amount of energy produced by wind and solar power plants as they are dependent on properties such as wind speeds, duration of sunlight and weather conditions which are beyond human control.
Therefore, to meet the energy demands of tomorrow, we need to devise energy storage methods for such sporadic energy production. Li-ion batteries are currently at the center of all the current advances in the battery industry and is widening its range of applications, but short life span and loss in charging efficiency are one of most dominant factors of not being able to use these batteries at a big scale.
This paper therefore, looks for solutions to store energy at a power plant level scale using Flow Batteries. Flow batteries are a cross between the traditional Li-ion and Fuel Cell batteries. With liquid electrolytes (catholyte and anolyte) being pumped through the electrodes, these batteries can maintain the same charging efficiency for a long time period.
The first ever Flow battery was developed in 1888 by Charles Renard to power 170-foot-long airship. Zinc chloride flow battery weighing about 450 kgs was used for this application. Vanadium Redox Batteries were invented approximately 100 years after the first flow battery. Today, most of the flow batteries have vanadium oxides as the electrolyte material. With emphasis on green chemistry growing, the search for materials which are reproducible and replenishable are desired. Even though, Vanadium is the 20th most abundant material in the earth’s crust, it is still present in a limited quantity.
The intent of this paper is to therefore look for materials which are reproducible and affordable. Organic materials are one of the areas which is still not explored to the limit. Due to high tunable properties of organic molecules, changing certain parameters such as solvent of electrolyte, electrode material, symmetry of organic solution and presence of membrane can lead to changes in the capacity as well as life span of the battery.
Creating Organic Redox Flow Battery (ORFB) can have many benefits in tackling the issue of disposal of batteries and replacing toxic materials with less hazardous materials. The applications of such batteries will not only be limited to large scale Solar and Wind energy storage, as these can also be used to power small to microgrids and can power electric vehicles of the future. Other than this, the research can also help in development of organic supercapacitors.
Introduction
Since the turn of the century, the search for a green, sustainable, renewable source of energy has escalated. Fossil fuels are being replaced with Solar and Wind Power, reducing the total carbon emissions to the atmosphere, and as a result, the cost of setting up a solar or wind power plant or a microgrid for both has gone down by a significant amount. Today, it is estimated that Solar energy will be responsible for 36% of the total energy produced in 2030. Although these alternative sources are becoming cheaper, the issue of fluctuation in energy output and sporadic nature of these resources has posed a challenge to scientists and engineers around the world.
As a solution to this problem, various energy storage devices such as lithium-ion batteries, fuel cells, and thermal storage devices are being investigated. Li-ion batteries can work for small scale storage like in mobile phones or automobiles, but for consistently powering the whole city of Philadelphia, we need a solution that can store large amounts of energy for a longer time period. Flow Battery could be a possible answer to this problem.
 Flow Battery is like a hybrid of Fuel Cell and a traditional Li-ion Battery. It works on the concept of pumping liquid electrolyte through electrode cores which are separated by membrane (see Fig.1). The battery consists of two tanks of an electrolyte liquid, one positive and one negative. At the middle of the tanks is a cell stack where the positive and negative solutions are pumped but kept separated by a membrane. The negatively charged solutions gives up electrons (Oxidation) and when connected through a device these electrons travel to the positive side where reduction takes place, making the complete cell a Redox cell. Ion exchange between the electrodes takes place when H+ ions move from positive cell to the negative through a membrane which allows ions transfer. The liquid electrolyte which connects with the cathode is called catholyte and similarly, we have anolyte. With this paper I would like to present my idea to overcome the current challenges faced in this sector.
Flow Battery is like a hybrid of Fuel Cell and a traditional Li-ion Battery. It works on the concept of pumping liquid electrolyte through electrode cores which are separated by membrane (see Fig.1). The battery consists of two tanks of an electrolyte liquid, one positive and one negative. At the middle of the tanks is a cell stack where the positive and negative solutions are pumped but kept separated by a membrane. The negatively charged solutions gives up electrons (Oxidation) and when connected through a device these electrons travel to the positive side where reduction takes place, making the complete cell a Redox cell. Ion exchange between the electrodes takes place when H+ ions move from positive cell to the negative through a membrane which allows ions transfer. The liquid electrolyte which connects with the cathode is called catholyte and similarly, we have anolyte. With this paper I would like to present my idea to overcome the current challenges faced in this sector.
Fig.1 Representation of a Redox Flow Battery using Vanadium ions. (Reference 1)
The most popular type of Flow Battery is the Vanadium Redox Battery (VRB) which uses vanadium ions dissolved in aqueous sulfuric acid as the electrolytes. The multiple valence states of Vanadium (V+, V+2, V+3, V+4) make this type of battery the most popular, as the battery capacity does not decrease when positive and negative solutions are mixed as there is no issue of cross contamination between the two sides of the membrane. This property allows the battery to continue for a longer lifespan. When compared to other electrochemical storage techniques, VRBs allows for sizing power and energy storage capacity independently (which is like Fuel Cells). This results in storing unlimited capacity of energy by just increasing the size of the tanks, thus, Redox Flow Batteries are scalable in nature.
Use of Vanadium solutions has also shown a capacity of continuing for more than 10000 charging cycles which is far greater than the current Li-ion battery and solid-state batteries rated lifetime. Another advantage of using such types of batteries is that they do not require much maintenance and are quickly charged by just changing the solutions. VRBs are also safer than the typical Li-ion Battery as they nonflammable in nature.
Because of its various advantages over other storage systems, companies such as Avalon Battery, Vionx, UniEnergy technologies and others are involved in funding and development of such batteries in the United States. There is also a Flow Battery based storage plant being set up in the Dalian province of China which will be the biggest energy storage plant in the world, having a capacity of about 200 MW/800 MWh energy storage. This industry is supposed to grow on to have a $ 4.5 bn market by 2028.
But Vanadium is only present in some concentrated regions mainly of China and Russia, and 90 percent of its extracted quantity is used up by the steel manufacturing industry, making Vanadium as a costly product. The price of the Vanadium metal is increasing, and it is expected to increase more when more energy storage devices utilize this material. Another challenge is the poor energy to volume ratio. For a battery producing KWs of power we need to build approximately a 40-foot large structure. This makes this type of system not suitable for small devices. But a major concern for these batteries are the toxic nature of the highly acidic Vanadium oxides. Therefore, we need to look for better, cheaper materials which fits the principles of green chemistry.
Addressing to the challenge of ease of availability of material, we can try other electrolytes consisting Fe or Cr, which also can exist in multiple valence states. But past studies have shown that they have reduced efficiency – 97% when compared with Vanadium’s 100%, as it need highly acidic solutions which reduces the columbic efficiency due to high exchange of H+ ions and exhibit high cross contamination between the two sides of the membrane during their operation.
Various combinations are being tested such as Zinc-Bromine Flow Battery by Primus Power and there is also ongoing research being done to combine organic materials with inorganic compounds of Vanadium and Iron. These batteries provide good charge densities and discharging capabilities but have their own cons like dendrites formation in the case of Zinc, therefore, requiring high maintenance.
In my view, using organic materials instead of rare earth metals/inorganic compounds would be a better and greener way to move forward in this technology. Using redox active materials which can undergo rapid and reversible redox reactions and whose properties can be tuned by changing their ambient conditions would be the perfect materials.
As shown from a research led by Michael Aziz et al, use of quinone structures which are redox active materials is possible in manufacturing of Organic Redox Flow Batteries (ORFBs). Quinone groups provide two electron, two proton redox reactions and changing their radical groups can affect the current densities of the cell. The findings of the study proved it is possible for an organic molecule to generate higher cell voltage values (typically >1.5 V as opposed to the VRBs which exhibits maximum cell potential of 1.5 V). And the organic materials are also cheaper than the other systems (as shown in the Table 1). The challenge in these types of systems is to increase the stability of the organic solutions that we use for a higher no. of cycles.

Table 1. Different types of Redox Active Materials compared on various parameters required for Energy storage (Reference 9)
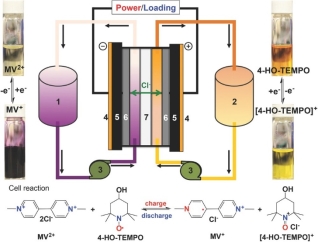 Other than quinone groups, we can also look for polymers with -O, -S and -Cl compounds which can produce different ionic states, such as MV/TEMPO combination as anolyte and catholyte of the battery. (See Fig 2).
Other than quinone groups, we can also look for polymers with -O, -S and -Cl compounds which can produce different ionic states, such as MV/TEMPO combination as anolyte and catholyte of the battery. (See Fig 2).
(a)
(b)
 Fig. 2. (a) Anthraquinone Redox Flow Battery (Reference 7) (b) MV/TEMPO aqueous redox flow battery (Reference 6)
Fig. 2. (a) Anthraquinone Redox Flow Battery (Reference 7) (b) MV/TEMPO aqueous redox flow battery (Reference 6)
Companies such as Jena Batteries are involved in research and development of such organic electrolyte-based batteries. They are using aqueous base solution of organic molecules to currently develop 30 KW/100 KWh battery to store electricity from renewable sources.
Furthermore, researches at various universities and laboratories are directed at observing the effect of dissolving these organics molecules in a nonaqueous solution. We can test possible variations of compounds with highly acidic and highly alkaline solutions by changing the molar concentration of the substrate. Using molecules such as anthraquinones which are naturally found in rhubarb will be helpful in reducing the synthesis cost factor of the device.
In the past researches, the organic molecules are tested with carbon electrodes. I think we will benefit from changing these electrodes to graphene-based oxides nanoplatelets. GNOPs has been previously studied in combination with Vanadium oxides and it helped in increasing the reversibility of the reactions. This can help in attaining stability for higher number of cycles and therefore will make these systems comparable with VRBs.
If we can optimize the combination of the catholyte and anolyte in such a way that it provides a symmetry of reactions on both sides of the membrane, we can increase the lifespan of the battery even more. For this we need organic molecules which are similar on both sides of the membrane and does not cross contaminate the solution. This can then help in large scale production of such cells which we can then stack to size the battery as per our usage.
Papers have come up in which scientists talk about achieving 60C charging capability at an efficiency of 60% using organic molecules. Research in this area to increase the efficiency of the battery for low charging times can be very fruitful. This can help in scaling down redox flow batteries for use in heavy usage devices which require high number of charging and discharging cycles to function such as electric cars, mobiles etc.
We also need to come up with membranes which are selective to the exchange of ions and solution. This is important as a highly permeable membrane can lead to high cross mixing of solutions which can slow the process of charging and reduce the capacity of battery over time.
For synthesis of such molecules, we can seek new methods of manufacturing such as self-assembly which will be very beneficial in mass production of such batteries. Research done in the area of self-assembly of quinone with toluene after the application of light can be tested for development of TEMPO or Anthraquinone structures.
The government of United States and the Department of Energy are funding various projects which are targeted at achieving high storage capacities for renewable resources. If this idea to develop large scale yet cheaper batteries is approved, we can look forward to tests our results on a bigger scale than the conventional method of testing at the coin cell level and can verify the previous research and our findings at a functional level. This can confirm the feasibility of such batteries which can power the whole city. Once proper research is done and experiments are performed, we can also involve commercial companies like SolarCity for the solar grid energy storage.
Benefits
Creating a battery out of redox active organic material can solve many challenges of the traditional Vanadium Redox batteries. Organic molecules such as Anthraquinones can be found in the nature and therefore have lower material costs even when compared to Li-ion batteries. There are researches done by scientists in USC where they have developed Organic Aqueous based Redox flow batteries which costs 1/10th the cost of Li-ion batteries of same capacity.
Finally, success of this research depends on the improvements in performance and capacity of such batteries when compared with VRBs or Fuel Cells. If successful in creating a battery using organic molecules which can be easily derived from nature, many sectors can benefit from our research. Other than developing large scale battery plants for storage and transmission of Solar and Wind energy, these batteries or organic molecules can have impact in areas such as
- Development of Supercapacitors – Currently supercapacitors uses graphene as their most basic component. Using redox active organic molecules can help in reducing the toxicity and cost of development of such systems.
- Development of small-scale batteries – Creating micro flow batteries whose moving parts can be replaced using capillary forces at the small scale, we can develop power units which can be easily transported, and which are also very thermally stable. Our mobiles and laptops can use such batteries, as micro organic redox flow batteries can help in maintaining low heating of our devices.
- With low charging/discharging time, these batteries can be used to power automobiles in the future and companies such as Tesla, Chrysler can benefit from our research. Issues of fires encountered with Li-ion batteries can also be tackled with ORFBs which use nonflammable materials and are safer.
- Development of single house micro grid – We can employ ORFBs for house applications, with capacities of about 10KWh of energy. Companies like Redflow, Tesla can profit from our research. Also, the house will be free from any toxic materials which require monitored waste disposal.
I think people living in areas such as California, where there are incentives provided by the government to households who can store and distribute renewable energy will hugely benefit for such systems. As these systems can have a lifetime of about 15 years, the overall initial investments can be generated back by selling the electricity produced and when there is a need to increase the storage capacity, we just need to build a bigger tank for the electrolytes. These batteries will also be helpful in the event of power outage or load shifting.
One day, Organic Redox flow batteries will have a huge role in the development of green energy grids. They fulfil the philosophy of affordable technology yet being coherent with the green engineering principles of less toxic materials and use of renewable sources to manufacture products.
References
- How does a flow battery work?
https://batteryuniversity.com/learn/article/bu_210b_flow_battery
- “Redox flow batteries for the storage of renewable energy: A review” by P Alotto et al
- China’s biggest flow battery project – https://www.energy-storage.news/news/chinas-biggest-flow-battery-project-so-far-is-underway-with-hundreds-more-m
- “Alkaline quinone flow battery” by Michael Aziz, et al
- “Graphene oxide nanoplatelets as excellent electrochemical active materials for VO2+/ VO+ and V2+/V3+ redox couples for a vanadium redox flow battery” by P Han, H Wang et al.
- “A Total Organic Aqueous Redox Flow Battery Employing a Low Cost and Sustainable Methyl Viologen Anolyte and 4‐HO‐TEMPO Catholyte” by T Liu, Wei Wang et al.
- “High-Performance Aqueous Organic Flow Battery with Quinone-Based Redox Couples at Both Electrodes” by
- Jena Batteries – https://jenabatteries.com/
- “Materials and Systems for Organic Redox Flow Batteries: Status and Challenges” by David Reed, Wei Wang et al.
- “An Inexpensive Aqueous Flow Battery for Large-Scale Electrical Energy Storage Based on Water-Soluble Organic Redox Couples” by G K Suryaprakash et al.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


