Anti-Corrosion in Marine Surface Engineering
| ✅ Paper Type: Free Essay | ✅ Subject: Engineering |
| ✅ Wordcount: 4336 words | ✅ Published: 30 Aug 2017 |
- INTRODUCTION
Surface building alludes to an extensive variety of advancements that plan to outline and change the surface properties of parts. There are two fundamental classes of surface designing techniques that can be utilized to advance the surface properties and the mass materials. These are surface coatings and surface Modifications. Other process include Surface shape design.
Surface covering forms include protecting a layer of liquid, semi-liquid or substance material onto a substrate. One of the fundamental elements of surface covering is to change and fortify the surface capacities as opposed to improving the arrangement of the mass material. A few cases of surface covering forms incorporate Physical Vapour Deposition (PVD), Concoction Vapour Deposition (CVD), plasma and warm splashing, sol-gel, cladding and electroplating. Surface modifications procedures can be named solidifying by fire, acceptance, laser or electron bar, high vitality medications, e.g. particle implantation; and dispersion medicines, e.g. carburizing and Nitriding. Surface adjustment procedures are relevant to control rubbing, enhance surface wear and consumption resistance, and change the physical or mechanical properties of the part. Surface alteration medications additionally can be consolidated with surface covering forms, for example laser cladding. This blend improves the benefits of surface coatings and surface adjustment, consequently accomplishing particular prerequisites and wellness for reason.
Surface medicines that cause microstructure changes in the bulk material incorporate warming and cooling/extinguishing through acceptance, fire, laser, and electron bar strategies, or mechanical medications (one illustration is icy working). Surface medicines that modify the science of a surface incorporate carburizing, Nitriding, Carbo-Nitriding, Nitro carburizing, boriding, siliconizing, chromizing and aluminizing
“Hard facing” is another type of surface treatment, where the mass material surface is given a defensive layer of another material having more predominant properties than those of the mass material. An example of this is covering a turbine pump seal joint with a destructive resistive material, to keep salty water from disintegrating the pump. Each strategy for hard confronting, cases of which are covering affidavit, cladding or welding, causes specific physical and concoction impacts on the mass material, some advantageous, some adverse. For instance focuses on which may exist in the defensive material can make issues, however watchful checking and research may restrain these impacts, to ideally create quality, serviceable building parts. The accompanying areas will portray the idea of surface building and the impacts designing situations have on these surfaces. Hard confronting procedures are portrayed in details particularly as to covering statement advances, with specific accentuation on warm splash strategies, including the HVOF (High Velocity Oxy-Fuel) prepare that utilized as a part of the ebb and flow inquire about.
1.1 Surface Engineering
At the point when two surfaces come into contact and relative movement is created, the contact stresses increased because of the generally little rate of load-supporting region. This will result in grating and wear, and potentially even prompt to disappointment. In the high anxiety utilizations of a present day motor or gearbox, there are zones where the weight of metal moving against metal powers away all the greasing up oil and permits warmth to develop. In the instance of extraordinary weight and contact, this warmth is sufficient to momentarily weld the two sections together just before they are broken separated by their development.
This consistent weld and break handle which can happen at the nuclear scale bring about drag and wear, which are damaging. On the off chances that the riggings are the parts of a shuttle, and if the single part comes up short, the whole multi-million dollar shuttle would fizzle. So designed surfaces to battle grating and diminish wear is exceedingly alluring.
Many materials have been developed to have specific bulk properties, although they have not been particularly optimized for the surface properties. Surface engineering can solve these problems by:
- Implanting alloying atoms to different depths, thereby improving toughness and fatigue properties; (surface modification)
- Depositing surface layers, thick or thin, including lubricants; (surface coating)
- Redesigning the surface shape of the component to distribute stresses.
1.2 Friction, Corrosion, wear
Surface engineering techniques solve friction, corrosion, and wear problems. Friction, corrosion and wear are the most common factors that cause engineering failures. In industrialized countries, 7% of GNP represents the cost of friction, wear and corrosion, with the possibility of 1% of this figure being reduced through the use of efficient tribological systems.
On a worldwide level, 34% of all lubricants produced are consumed in Europe, with 50% of this being recycled, with a further 2.5 million tonnes being lost in the environment. Corrosion is mainly an electrochemical phenomenon which occurs when metals react with materials in their environment.
1.3 Basic types of Corrosion and wear
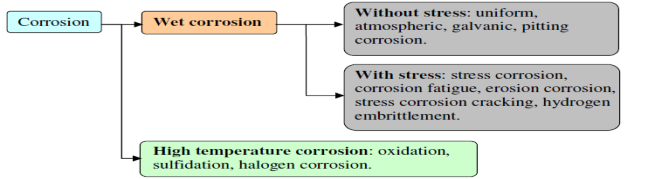
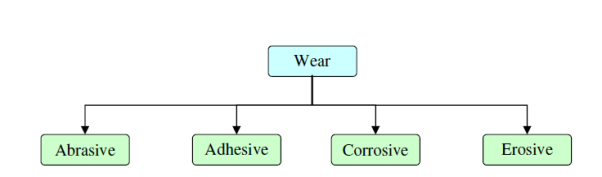
Fig. 1: Type of corrosion and wear
Wear is the dynamic loss of material from a surface. Among the distinctive sorts of wear, rough wear and glue wear ordinarily happen more frequently than others. Grating wear is because of hard particles or hard bulges constrained against and moving along a strong surface. Cement wear is created by restricted holding between reaching strong surfaces prompting to material exchange between the two surfaces or the misfortune from either surface. Cement wear is a run of the mill case of how a delicate material can wear out a harder on.
1.4 Surface Engineering in Marine applications

Methods of corrosion protection are based on the theory of corrosion processes. Alterations of the material properties and external environmental characteristics are the factors which can affect the slowing down or stopping of the corrosion process. Corrosion protection in the shipbuilding industry is an important technological process devised to protect the ship from corrosion effects. Even the storage area of plates, profiles and flats in a shipyard exposed to severe environmental conditions.
Protection Coating in shipbuilding constitutes of following steps:
- Preparation of surface
- Temporary protection workshop
- Terms of coating
- Methods of Applying coating
1.5 Preparation of Surface
Surface arrangement is a requesting innovative operation in the advancement of the ship’s body from essential surface readiness of plates, profiles, and pads performed in the programmed establishment .It likewise incorporates a fundamental workshop technique called covering. Shop groundwork in the film thickness 15-25 μm is utilized for the assurance of steel during the time spent ship development. In any case, compulsory control of the thickness of the coatings for more prominent thickness may unfavourably influence the cutting pace and nature of steel, and in addition the event of mistakes in welding.
Optional surface planning is done in the corridors for sand impacting and painting, whereby soil and salt ought to be expelled from the surface of the area, rendering it and sandblasting as per standard ISO 8501-1. This innovative operation infers additional work in welding and crushing and speaks to the pre-gathering attachments.
1.5.1 Temporary protection of surface
The principal phase of consumption assurance in the shipbuilding business comprises of the workshop covering (shop groundwork) on the plating profile and pads in a thin film, about of 15 to 25μm thick, as intemperate film thickness may adversely influence the nature of welding (the event of porosity) and cutting steel. Shop preliminary speaks to quick drying coatings which are utilized for transitory security of steel in the workshop amid ship development and utilization of the last covering framework. Transitory erosion security is performed in the workshop of preparing in mechanized plant beginning from drying sheets, shot impacting and covering as indicated by the ISO standard.
Workshop coatings utilized are zinc silicate and epoxy press oxide shop preliminary. Epoxy press oxide is connected to acquire a more prominent thickness, requesting more paint, thusly bringing about higher coats and expanded misfortunes. Today in many shipyards zinc silicate is utilized as shop preliminary (with a share of 25-35 % zinc), which gives a superior quality welding and lessened event of undesirable zinc salts (a compound barely solvent in water and hard to clean from the surface) Shop preliminary must meet the accompanying prerequisites:
- It should be appropriate for mechanized re-coloring methodology,
- It have the most limited drying time (3 to 5 minutes),
- Imperviousness to high temperatures,
- It shouldn’t discharge dangerous gasses amid welding and cutting,
- It shouldn’t adversely influence the welding procedure & It should not influence the mechanical properties of welded joints.
1.5.2 Terms of coating
Surface readiness principles incorporate a few criteria, tenets and rules for the way toward get ready body metal surfaces. Surface states of steel structures are isolated into four phases:
- Steel surfaces secured with plant scale and little erosion,
- Steel surfaces, which have started to erode and from which process scale has begun to peel
- Steel surface displaying particles of rust or inadequately connected worrying factory scale with the main noticeable sings of setting,
- Steel surface that is unmistakably consumed and influenced by setting.
The way towards planning metal surfaces done by different methods for abrasives. Abrasives are distinctive sorts of materials appropriate for the specific coarseness surface readiness, where the grating particles utilizing packed air are connected to the surface which is to be cleaned.
1.5.3 Method of Applying Coating
The innovative procedure of erosion security utilizes diverse techniques for applying coatings by brush, roller or showering (air or airless), where the determination of the methodology influences the speed and nature of works.
- Paint brush is normally utilized for the methodology “touch-up” in the insurance of zones, for example, joints, moves, edges, welds and other unpleasant surfaces and setting. In these regions, color entrance is accomplished by applying the correct brushes: it cannot be accomplished by whatever other technique.
- Paint roller is seldom utilized in light of its deformities, arrangement of little uneven layers, more often than not with little crevices and openings.
- Applying a covering by splashing depends on scattering the paint as small beads that settle on the working parts. Airless splashing is a strategy that is most basic in the shipbuilding business since it empowers quick use of Paint onto extensive surface, high effect, the likelihood of applying thicker layer, and Great infiltration.
- ARC SPRAYING PROCESS
Thermal spraying method is a process of coating in which a heated material is sprayed on its surface. This type of spraying can provide thick coatings over a large area at high deposition rate. Thermal spraying is classified into Plasma, Wire arc, Flame, Detonation, Warm and cold spraying.
2.1 Overview of Arc spraying process
In electric arc spray process, two wires of desired materials act as electrodes are fed to spray gun at controlled feed rate. Compressed gas is also used to atomize and propel the material to the substrate.
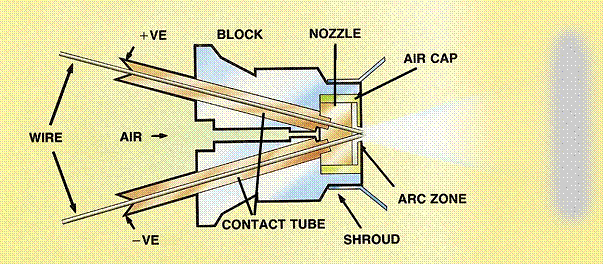
Fig.2: Schematic diagram of Wire arc spray device
In this process, the anode and cathode wires are continuously melted and broken down into tiny droplets which are injected by air jets through a nozzle. The velocity of gas is a few hundreds of m/s but the gas is not heated, allowing operator to keep the substrate temperature below a few 0C without cooling. The arc powers generally range from 2 to 10 KW, airflow ranges from 0.8 to3 m3/min. The temperature of the arc can be as high as 20,000 k. DC generators are used a source for power.
Before the arc process is performed, substrate surface is properly cleaned. For this purpose usually the surface is blasted using a compressed air abrasive blasting. This is effective for cleaning mill scaled on steel surface by using abrasive particle at high velocity and also for good adhesion of arc spray process. Along with this the substrate surface roughness is also important consideration to be made for proper coating bonding. To hinder rust formation and provide good bonding the coarse surface profile must be around 110 microns. Garnet is normally used abrasive as it can provide desired surface finish and roughness. As metallic abrasive usually result in energy being given off as a spark which might cause surface defect non -metallic abrasives are used.

Fig.3: Arc spray process instrument
Before the coating is applied on the substrate surface some conditions need to be satisfied, they are
- Surface finish must be greater than SA 2.5 as it can be visually inspected
- Surface roughness must range between 75 microns to 110 microns
- Permissible level for salt contamination of surface should not exceed 20 mg/m3
- Dust level should not exceed the rating 1
2.2 Characteristics of Arc spray process
- Wire diameter: The wire diameter ranges from 1.6mm to 3.16mm. The deposition rate of coating on the surface depends on the wire diameter.
- Wire feed rate: This determines the rate at which the material must be introduced to the system, which is proportional to the rate at which the material introduced melts.
- Coating materials: Metals, cements, ceramics, polymers, carbides are some of the materials used for arc spray process. Solid wires produced from Chromium, zinc nickel based alloys or aluminum are generally used for arc spraying in marine application. Aluminum is considered to have the best performance for surface coating.
- Spray distance: The distance varies from 15cm to 25cm; the distance affects the deposition area of coating on the surface.
- Power requirement: Depending on the spraying material, voltage ranges from 16 V to 30 V. The applied voltage controls the input power to the arc which affects the rate at which the tips of the wires are heated and melted. The current is adjusted to 150 A for most of the applications.
2.3 APPLICATION OF ARC SPRAY PROCESS
This process is mainly used for corrosion protection. Other than this, it is also applied for coatings of machineries like boiler tube, gear box, shafts and crankshafts in power plants.
2.4 ADVANTAGES AND DISADVANTAGES
2.4.1 Advantages
- Arc spraying process is economical when compared with other spraying techniques
- Any coating material can be used. Examples are ceramics, cement metals
- It has a high deposition rate of the thermal processes
- Operator requires less training
- The power requirement is also low
- Almost all substrate material can be coated
- Low porosity level can be achieved using this process
- It’s useful as low heating of substrate makes arc spraying process useful in metalizing thermally sensitive substrate
2.4.2 Disadvantages
- Large amount of fume and dust is produced, which is a cause of concern for the operators health
- The materials used are limited to electrical conduction
- It can be sometimes seen that the coating quality does not meet to expectations as done with other technique
3. HOT – DIP GALVANIZING
3.1 Overview of Hot Dip Galvanizing
This process is used for coating iron an steel with a layer of Zinc by immersing the metal in molten zinc bath at a temperature of about 449 oC. This process forms a metallurgical bond between steel and molten Zinc and is done in a plant with controlled conditions. The bond formed has very good corrosion resistance due to the good molecular adhesion and the cathodic protection.
The hot dip galvanization process mainly consists of 4 stages.
- Surface Preparation – Removing oil/grease, dirt, paint etc. from the surface.
- Pickling in Acid – To remove mill/scale.
- Flux Coating – (Usually Zinc Ammonium Chloride) To prevent the oxidation of cleaned surface when exposed to air.
- Zinc Bath – Dipping the material in Zinc bath and holding it until it reaches equilibrium with the bath temperature.
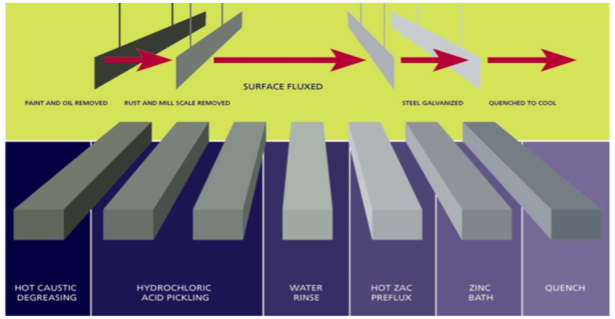 Fig.4: Various steps in hot dip galvanizing method
Fig.4: Various steps in hot dip galvanizing method
In HDG coating, the outermost layer (ETA) is a covering of pure zinc and is formed as the material is withdrawn from the molten Zinc bath. The underlying series of layers (of coating) are zinc-iron alloys and are formed as a result of metallurgical reaction between the molten zinc and the material being galvanized. In HDG process, the coating formed on the corners and edges of the material is generally thicker than the surrounding coating. The reason for this is that the zinc-iron alloys formed would grow perpendicular to the steel surface. The ETA layer is ductile and provides good impact resistance to the galvanized material. The combination of all the layers in the coating provides toughness to the material and also aids in resistance to mechanical damage in transport, erection and service of the components.
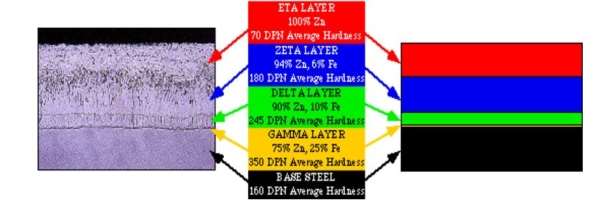
Fig. 5: Typical cross section of a hot dip galvanized coating
3.2 Variable Parameters
The appearance and the thickness of the galvanized coating are affected by several factors.
- Steel Composition: The galvanizing reaction produces thicker coatings for rough steel surfaces because of the increased surface area and also these coatings would have a poor appearance and would be a rough coating.
- Bath Immersion time: As galvanization is a diffusion process, the reaction between molten zinc and steel would be quick initially but slows down as the alloy layers grow and become thick. So, continuous immersion or dipping of the material more than once doesn’t produce a significantly thicker coating. (Which happens in case of reactive steels)
- Speed of Withdrawal: The withdrawal rate of material from the galvanizing bath has a significant effect on the outermost layer of the pure zinc. Thickest coating would be formed when the withdrawal is rapid because this allows a large amount of zinc to be carried out on the material which would eventually solidify and become pure zinc layer. On contrary, smooth, thin and uniform coatings are formed with slower withdrawal rates as it allows the zinc to drain back into the kettle.
- Steel cooling Rate: The dull or matte gray coatings are usually due to slower cooling rates such as air cooling or quenching of thicker sections because the slow cooling would allow the zinc-iron alloying reaction to continue and the inner alloy layers would use the outer ETA layer for their zinc supply. The formation of dull/matte surfaces can be reduced by halting the galvanizing reaction by quickly reducing the temperature to less than 300oF.
3.3 ADVANTAGES AND DISADVANTAGES
3.3.1 Advantages
- Lower first cost: This process generally has the lowest first cost compared to any other protective coatings for steel. The application cost of painting which is a labor intensive coating has risen far more than the cost of factory applied hot dip galvanizing.
- Lower maintenance / lower long term cost: In some cases, the initial cost of hot-dip galvanizing is higher than alternative coatings, but it is more cost effective due to its lower maintenance costs during long service life. Maintenance is costlier for structures in remote areas. Maintenance programs have an invariably negative impact on productivity.
- Long Life:In hot dip galvanized coatings, the life expectancy of structural members is 50 years more than in more rural environments and 10 to 30 years in most corrosive urban and coastal environments.
- Surface preparation:To ensure uniform cleaning of steel surfaces,they must be immersed in acid whereas organic coatings must be applied on abrasive blast cleaned surfaces and should be verified by third party inspection.
- Adhesion: This coating is metallurgical bonded to the steel surface.
- Environment friendly:The coating is non – toxic and doesn’t contain any volatile substances.
- Speed of coating application:A full coating can be applied in minutes whereas multi paint coating system takes up to a week. The application of hot dip galvanized coating is not influenced by weather conditions.
- Uniform protection:Hot dip galvanized surfaces are protected both internally and externally including recesses, sharp corners and inaccessible areas for application of other coating methods. The coating is at least as thick over sharp corners as on flat surfaces. No other coating applied can provide similar uniform protection.
- Sacrificial protection for damaged areas:A hot dip galvanized coating would preferentially corrode to steel providing cathodic protection to small areas of steel exposed to damage.
- Toughness:A hot dip galvanized coating has outstanding resistance to mechanical damage during transport, erection and service due to its unique metallurgical structure.
- Disadvantages
- This process can only be carried out in a galvanizing plant and on site application is not possible. The color of the zinc coating can only be changed by painting the coated surface.
- The size of the zinc bath is a limiting factor for the component or structure dimensions.
- There is probability of distortion/warp of large flat unsupported sheet components and long, slender beams due to the high molten zinc temperature.
- The welding of the zinc coated steel would need different procedures as compared to the uncoated steel. During welding of hot-dip galvanized steel, there is a loss of coating in the first and second heat affected zones, but a portion of the coating remains right up to the edge of the weld.
- COMPARISON OF ARC SPRAY PROCESS AND HOT DIP GALVANIZING FOR COATING OF SHIP HULL
As studied in the section’s before both Arc Spray Process and Hot Dip Galvanizing has their advantages and disadvantages. In this section we will compare both these process based on important characteristics and try to find out which one would be a better suitable process for coating of ship hulls.
4.1 Surface Preparation
Hot Dip Galvanizing requires specific surface preparation consisting of Degreasing, Pickling and in Flux solution, not only these surface preparation method requires special setup, but processes such as Pickling can introduce Hydrogen in the substrate causing Hydrogen embrittlement and Hydrogen Induced Cracking, however if the substrate steel is not cleaned properly the zinc will not bond with it and it may be noticed at that stage only. Surface preparation for Arc Spray requires the surface to be little coarse which can be done by abrasive air blasting which depends on the operators experience, but if the cleaning is not done properly it may not be detected then. Thus surface preparation by Hot Dip Galvanizing is advantageous.
4.2. Coverage
Arc spray process cannot be used for coating of corners, edges and complex shapes such as threads and hollow section, all of which can be coated easily by hot dip galvanizing as it is a dip process. Since ship hulls are large bulky object which doesn’t have any such complex shape both processes can be used here but still Hot Dip Galvanizing will be more beneficial.
4.3. Process Time and Economy
Hot Dip Galvanizing takes less time as it can coat almost all the surface area at one go when dipped inside the bath whereas Arc spray process is a line by line process and hence takes more time.
The equipment cost for arc spray is lower, its setup is also easier than Hot Dip Galvanizing, and the equipment is mobile whereas the baths for Hot Dip Galvanizing is stationary but this method can cover large area at one go. Therefore for coating of ship hull, Hot Dip Galvanizing is a faster process as they have a very large surface area for which Arc Spray process will take more time and the higher cost of Hot Dip Galvanizing is negated by ability to cover larger surface area at one goes.
4.4. Effect on Substrate
For Hot Dip Galvanizing the baths are maintained at a temperature of around 450°C which is just below the austenitizing temperature of steel and hence may cause a change in microstructure of the substrate if there are some variations whereas since no hot jet is directed on the substrate in the arc spray process there is no effect on the underlying substrate. Thus only considering this effect on substrate thermal arc spray process is a better process.
4.5. Coating Thickness
The thickness of coating in electric arc spray coating can be regulated by regulating the speed of wire feed in the process, thus it can be easily controlled whereas in Hot Dip Galvanizing a certain thickness may not be specified as the thickness depends on the withdrawal speed which may cause an uneven distributed coating.
4.6. Bond Strength
The bond strength for arc spray process for ferrous and non ferrous alloys is within the range of 4000-7000 psi whereas for Hot Dip Galvanizing the bond strength is around 3600 psi, thus arc spray process provides comparatively higher bond strength.
4.7. Hazard
Fumes and other hazardous by products are formed during the Hot Dip Galvanizing can pose health hazard to the operators who are working in the shop floor as well as to the environment. Thermal Arc Spray process on the other hand produces metallic dust consisting of very fine particles and fumes; also electric arc spray process operates at high current which can result in shock hazards if not treated with caution. Clearly both processes are hazardous both to human as well as environment.
4.8 Conclusion for comparison
Considering all the above factors Hot Dip Galvanizing seems to a better and efficient process for coating of ship hulls, not only coating ship hulls which have large surface area by arc spray a time consuming task it doesn’t providing any other significant advantages as compared to Hot Dip Galvanizing.
- CONCLUSION
In this report, we have mentioned the different possibilities for anti-corrosion in marine environment. Here we have taken the two commonly used methods for the protection of ship hull from corrosion.
As the instrument for arc spray process is quite cheaper compared to the other techniques, it’s widely used in marine industry. The coating adherence is good with no heating of the substrate generally; however coating done by this method is porous.
The hot -dip galvanizing process is an effective way for resisting corrosion. It is a simple process with better service life. It gives high quality with low environmental pollution making it a good competitive method.
After comparing the two methods, we can conclude that arc spray process is used if we want good control and for better quality, on the other hand hot-dip galvanizing is used for mass production as it turns to be economical.
6. REFERENCES
[1] Muhamad Hafiz Abd Malek, Nor Hayati Saad, Sunhaji Kiyai Abas, Noriyati Mohd Shah, “Thermal Arc Spray Overview”, IOP Conf. series: Material science and engineering 46 (2013), doi:10.1088/1757-899X/46/1/012028
[2] M.F.O Schiefler Fiho, A.J.A Buschinelli, F.Gartner, A.Kirsten, J.Voyer, H.Kreye, “Influence of process parameters on the quality of thermally sprayed X46Cr13 stainless steel coating”, COBEF 2003- Brazilian Manufacturing Congress, 18-21 May 2003, Vol.XXVI, pp 98-106
[3] Website ‘http://www.environment911.org/Environmental_Issues_With_Galvanizing‘
[4] Website ‘http://www.galvanizeit.org/education-and-resources‘
[5] Website ‘https://www.scientific.net/AMR.685.271‘
[6] Website ‘https://www.upc.edu/sct/en‘
[7] Website ‘http://www.mbicoatings.com/content.cfm/Coatings/Arc-Spraycoatings/ category id/102/page id/170‘
[8] Website ‘http://www.aeromac.com.sg/thermalsprayadv.html‘
[9]Website ‘http://www.metalplate.com/techdept/characteristics.php‘
[10] Website ‘https://www.canam-construction.com/wp-content/uploads/2014/12/2012-12-hot-dip-galvanization-of-structural-steel.pdf‘
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal



