Immune System Case Study
| ✓ Paper Type: Free Assignment | ✓ Study Level: University / Undergraduate |
| ✓ Wordcount: 2108 words | ✓ Published: 06 Jun 2019 |
Case study 1
A 3-month old baby presents with severe and persistent infections. He is not growing because of viral and parasitic (Cryptosporidium) gastroenteritis. He needs oxygen to breathe because of viral/parasitic pneumonia. He has fungal infection (Candida) in his mouth and his bladder.
- What major component of the immune system is important in host immunity to viruses?
Both the innate and adaptive components of the immune system are involved in viral immunity (1–3). Upon infection with viruses the innate immune system is activated through recognition of viral particles by pattern recognition receptors. Following recognition, the innate immune system produces IFN-α and β, cytokines required to activate NK cells. NK cells then kill virally infected cells. The innate response is also critical for activation of the adaptive immune system, which plays a major role in host immunity to viruses. The adaptive component of the immune system can be split into cellular and humoral responses, both of which are required for viral immunity. The humoral response is the production of antibodies which bind to virus particles circulating in the blood, preventing the spread of infection. The cellular response is T-cell mediated with CD4+ T-cells required for lymphocyte maturation while CD8+ T-cells are essential for killing virally infected cells (1–3).
In addition to preventing spread of viral infection and death of infected cells the adaptive immune response has a major role in memory responses, which produce swift and specific responses upon re-infection. Memory responses are less reliant upon the innate system recognising viruses, and thus provide immunity to viruses (2). Upon first infection with a virus the adaptive response, termed here the primary response, may take days to occur. In cases of reinfection with a virus the memory response, also known as secondary response, can occur within hours. A memory response is mediated by both B- and T-cells through a lymphocyte subset termed memory cells. These memory cells can survive for several years within an individual and, upon reinfection, can initiate a secondary response that is often more effective than the original primary response.
Therefore, while the innate component of the immune system is required for defence against first exposure to a virus, T-cells are important for long-term host immunity to viruses.
- What component of this infant’s immune system may be missing?
Given the breadth of pathogen’s present, and considering the child’s age, the immune system component missing in this infant is T-cells. The T-cells may be low in numbers or completely absent, or present but non-functioning.
- What simple laboratory tests would help to work out what is wrong with this child?
The clinical picture suggests that this patient has a cellular immunodeficiency such as severe combined immunodeficiency (SCID), Omenn’s syndrome or CD40L deficiency or is infected with HIV (4–6). Laboratory tests that would aid in the diagnosis of this child include:
- Full blood count used as a broad screening test to check for such disorders as anaemia, infection, and many other diseases.
- Absolute lymphocyte count (T, B and NK cells) – measures percentages and absolute numbers of lymphocytes, CD3 T cells, B cells and NK cells. Can identify which component of adaptive immune system is missing in an immunodeficient patient.
- Immunophenotyping – measurement of lymphocyte activation (CD3/HLA-DR, CD45 RA/RO ratio) and TCR phenotype analysis can aid in diagnosis. HLA-DR expression on CD8+ T-cells has been shown to be increased in HIV infection while high numbers of γδ+ T-cells or CD45 RO+ lymphocytes are suggestive of Omenn’s syndrome (7).
- Immunoglobulin levels – immunodeficient patients may have markedly reduced or absent immunoglobulins (IgG, IgM and IgA). A caveat to this test in this case is the age of the child may make results difficult to interpret.
- CD40L assay – if T-cell numbers are normal a CD40L assay may aid diagnosis of Hyper IgM syndrome, where T-cells are non-functional. CD40L deficiency leads to very low or absent expression of CD40L on activated CD3 T-cells.
- T-cell proliferation assay – T-cells of healthy individuals will proliferate upon stimulation, and flow cytometric analysis reveal a decline in CFSE staining within the stimulated cells, as the dye is divided equally with each cell division. However, this is not observed in those patients whose T-cell proliferation is impaired.
- HIV testing.
- What general treatment options are available for treatment of this patient?
Individuals with cellular immunodeficiencies are susceptible to deep-seated bacterial infections, viral and fungal infections, tuberculosis and other mycobacterial infections (8,9). Treatment options for this patient will depend upon the final diagnosis as follows:
Treatment for SCID/Omenn syndrome includes use of supportive therapies such as antimicrobials and intravenous immunoglobulin (IVIG, replacement antibodies) until a haematopoietic stem cell transplant (HSCT) or gene therapy can be performed (4).
CD40L deficiency is treated with Septrin® (co-trimoxazole), a prophylactic against Pneumocystis jiroveci pneumonia, and IVIG. A HSCT is currently the only cure (5).
HIV infection will require treatment with HAART (highly active antiretroviral therapy) and antimicrobials.
Case study 2
A 16-month old infant presents with severe disseminated mycobacterial infection and was previously treated for a Cytomegalovirus infection. The child has fever, sweating and diarrhoea, and abdominal tenderness and lymphadenopathy on examination.
- What component of the immune system may be defective?
Mycobacteria are targeted by macrophages, an essential component of the innate immune system (2). However, to effectively remove a mycobacterial infection, macrophages require CD4+ T-cells (known as T helper cells) for activation via the IFNγ/IL-12 pathways (2). Thus, this infant with severe disseminated mycobacterial infection may have a deficiency in either their innate immune system or in their T-cells.
- Discuss likely diagnoses from the clinical picture.
Disseminated mycobacterial infections are associated with the following disorders (10,11):
- Mendelian susceptibility to mycobacterial disease (MSMD),
- HIV infection,
- Idiopathic CD4 lymphocytopenia,
- T-cell immunodeficiencies such as combined immunodeficiency (CID),
- Lymphoid malignancies.
Severe disseminated mycobacterial infection, in association with the clinical features of fever, sweating and diarrhoea, are diagnostic of MSMD (12). Diffuse abdominal tenderness and general lymphadenopathy are also characteristic of this disorder (12).
- What are the associated pathways involved?
An immune response against mycobacteria and salmonella require appropriate interactions between phagocytes and Th1 cells. The IL-12/IFNγ pathway is required for appropriate activation of phagocytes following infection with mycobacteria or salmonella (3). Defects in the phagocyte/Th1 or IL-12/IFNγ pathways are associated with MSMD (11–13). When phagocytes such as dendritic cells and macrophages are infected with mycobacteria they respond by producing and secreting IL-12. This IL-12 secretion stimulates CD4+ T-cells and NK cells through their IL-12 receptor, resulting in the production of IFN-γ (2). IFNγ binds to the IFNγ receptor (IFNγR) on the phagocyte leading to signalling through the JAK/STAT pathway which in turn leads to the transcription of IFNγ regulated genes, one of which produces TNF-α (13). TNF-α is an inflammatory cytokine that is involved in a diverse number of signalling pathways that lead to cell death through necrosis or apoptosis. In addition, IFNγ promotes mycobacteria death through an as yet unidentified mechanism.
- Discuss how signalling would normally protect the patient, and how the defect impacts the immune response.
There are currently 9 genetic defects identified within the IL-12/IFNγ pathway associated with MSMD (12). Defects have been identified in seven autosomal genes (IFNGR1, IFNGR2, STAT1, IL12RB1, IL12B, IRF-8, ISG15) and in two X-linked genes (IKBKG/NEMO, CYBB). These defects affect the pathway at several different points (Figure 1).
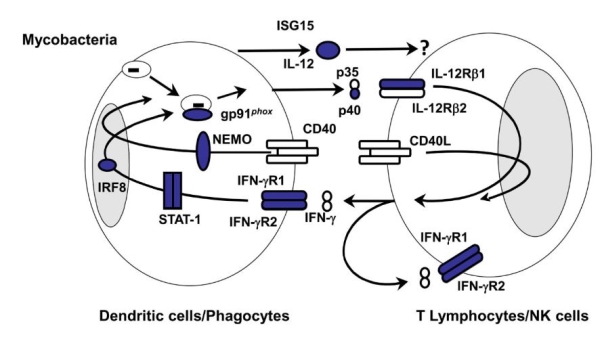 Figure 1: Diagrammatic representation of the IL-12/IFNγ pathway. Areas in blue indicate known defects. Image taken from (13).
Figure 1: Diagrammatic representation of the IL-12/IFNγ pathway. Areas in blue indicate known defects. Image taken from (13).
The IFNγ receptor (IFNγR) is composed of two chains, IFNγR1 and IFNγR2. If a defect is present in either of these chains then a complete or partial deficiency in the IL-12/IFNγ pathway will occur. This causes defective signalling via IFNγR due to loss of receptor expression. Defective signalling via IFNγR results in a loss of IL-12 and TNF-α production meaning the infection is not removed (13,14).
STAT1 (signal transducer and activator of transcription 1) is involved in cellular responses mediated by IFNγ and mutations in STAT1 can cause loss- or gain-of-function. A loss of STAT1 function results in an impaired ability of infected cells to respond to IFNγ resulting in a susceptibility to mycobacterial and viral infection. In a gain-of-function mutation infected cells have a stronger response to IFNγ with no susceptibility to viruses (13,14).
CYBB is a significant element of the NADPH complex expressed in phagocytes. Mutations in CYBB are most commonly associated with chronic granulomatous disease where mycobacterial infections are not a common clinical feature. In phagocytes infected with mycobacteria, most commonly macrophages, MSMD-associated CYBB mutations appear to inhibit the respiratory burst function that is crucial in providing macrophages immunity to mycobacteria (13).
The known IL-12 deficiency (IL12B, IL-12p40) is a loss-of-functionmutation. IL-12p40 is common to both IL-12 and IL-23. IL-12 binds to IL-12R and induces IFNγ secretion while IL-23 binds to its receptor and induces IL-17 secretion. Individuals with this mutation have a milder clinical phenotype compared with IFNγR defects. While IL-12 secretion does not occur in affected individuals, IFNγ secretion may still occur, albeit at lower levels, via IL-12 independent pathways (11,13).
As with the IFNγ-R, the IL-12R is a heterodimer (IL-12-Rβ1, IL-12-Rβ2). Defects have been identified in one of the chains, IL-12Rβ1. The identified defects cause premature stop codons resulting in a reduced expression of the receptor at the cell surface resulting in defective IL-12 signalling and a reduction in IFNγ secretion. ISG15 induces production of IFNγ by T-cells and NK cells. The clinical picture in patients with ISG15 mutations is similar to that observed in those with IL-12p40 or IL-12-Rβ1 defects with a reduction in IFNγ production, but not complete loss (11,13,14).
NEMO is a regulatory subunit of NF-kB kinase (IKK) and is not a direct part of the IL-12/IFNγ pathway. Mutations in NEMO have been associated with several disorders including anhidrotic ectodermal dysplasia with immunodeficiency, incontinentia pigmenti and MSMD. NEMO mutations associated with MSMD are thought to impair CD40-dependent IL-12 production, leading to a reduction in IFNγ production (13).
IRF8 (interferon regulatory factor 8) is a member of the IRF family and is expressed by macrophages and dendritic cells. A complete deficiency in IRF8 is associated with loss of localisation and transcriptional activity leading to an impairment of IL-12 and IFNγ production (13).
In summary, mutations in IFNGR1, IFNGR2, STAT1 and CYBB impair the action of IFNγ while mutations in IL12B, IL12RB1, ISG15 and NEMO impair the production of IFN-γ. IRF8 mutations affect both production and function of IFN-γ.
References
1. Mueller SN, Rouse BT. Host defenses to viruses. In: Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew, Anthony J, Weyand CM, editors. Clinical Immunology: Priniciples and Practice [Internet]. Fourth. elsevier; 2013 [cited 2018 Feb 22]. p. 346–55. Available from: https://www.sciencedirect.com/science/article/pii/B9780723436911000179
2. Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 8th International. Philadelphia: Saunders; 2015.
3. Delves PJ, Martin SJ, Burton DR, Roitt IM. Roitt’s Essential Immunology. 12th ed. Oxford: Wiley-Blackwell; 2011.
4. Bonilla FA. Severe combined immunodeficiency (SCID): an overview [Internet]. Stiehm ER, editor. UpToDate. 2017 [cited 2018 Mar 2]. Available from: https://www.uptodate.com/contents/severe-combined-immunodeficiency-scid-an-overview
5. Notarangelo LD. Hyperimmunoglobulin M syndromes. In: Stiehm ER, editor. UpToDate [Internet]. 2017 [cited 2018 Mar 16]. Available from: https://www.uptodate.com/contents/hyperimmunoglobulin-m-syndromes
6. Rezaei N editor. Primary Immunodeficiency Diseases Definition, Diagnosis, and Management. Aghamohammadi A editor, Notarangelo LD editor, service) S (Online, editors. Berlin, Heidelberg : Springer Berlin Heidelberg,; 2008.
7. Kestens L, Vanham G, Vereecken C, Vandenbruaene M, Vercauteren G, Colebunders RL, et al. Selective increase of activation antigens HLA-DR and CD38 on CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–41.
8. Gennery A. Recent advances in treatment of severe primary immunodeficiencies [version 1; referees:2 approved]. F1000Research. 2015;4(F1000 Faculty Rev):1459.
9. Subauste CS. Primary immunodeficiencies and susceptibility to parasitic infections. Parasite Immunology. 2006.
10. Lee WI, Huang JL, Yeh KW, Jaing TH, Lin TY, Huang YC, et al. Immune defects in active mycobacterial diseases in patients with primary immunodeficiency diseases (PIDs). Journal of the Formosan Medical Association. 2011.
11. Picard C, Puel A, Bustamante J, Jouanguy E, Zhang S-Y, Dupuis-Boisson S, et al. Inherited disorders of IFN-γ-, IFN-α/β/λ-, and NF-κB-mediated immunity. In: Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew, Anthony J, Weyand CM, editors. Clinical Immunology: Principles and Practice [Internet]. Fourth. Elsevier; 2013 [cited 2018 Feb 22]. p. 454–64. Available from: https://www.sciencedirect.com/science/article/pii/B9780723436911000209
12. Uzel G. Mendelian susceptibility to mycobacterial dieases: An Overview. In: Puck JM, editor. UpToDate [Internet]. 2017 [cited 2018 Mar 2]. Available from: https://www.uptodate.com/contents/mendelian-susceptibility-to-mycobacterial-diseases-an-overview
13. Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Seminars in Immunology. 2014.
14. Uzel G. Mendelian susceptibility to mycobacterial diseases: Specific defects. In: Puck JM, editor. UpToDate [Internet]. 2017 [cited 2018 Mar 2]. Available from: https://www.uptodate.com/contents/mendelian-susceptibility-to-mycobacterial-diseases-specific-defects
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this assignment and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


