Slowing Osteoarthritis Progression in a Rat Model of Posttraumatic Osteoarthritis
Info: 4292 words (17 pages) Dissertation
Published: 24th Feb 2022
Tagged: Biomedical Science
Abstract
Current available treatments for osteoarthritis (OA) are restricted to analgesics such as NSAIDs which may cause severe adverse effects after prolonged consumption. These pharmacologic treatments provide pain relief but cannot arrest or slow progression of OA. Nutraceuticals are food products that offer favorable safety profiles for long-term use and may exert disease- and symptom-modification effects in OA by suppressing activity of pathways involved in OA pathogenesis such as inflammation and catabolic activity. Drug N is a patent-pending nutraceutical formulation that synergistically targets these OA pathways. This study aimed to determine whether Drug N slowed progression of posttraumatic OA in a rat model of destabilization of the medial meniscus (DMM). Compared to placebo- and ibuprofen-treated DMM rats, Drug N-treated DMM rats exhibited:
(1) reduced proteoglycan loss, cartilage erosion, and cartilage fibrillation,
(2) lower OARSI scores,
(3) reduced immunohistochemical staining of type II collagen and aggrecan cleavage epitopes,
(4) reduced levels of proteolytic enzymes Mmp13 and Adamts5, pro-inflammatory cytokines Il1b, Il6 and Tnfa, and increased expression of Cited2.
This study provides the first direct evidence that Drug N is effective in slowing disease progression in moderate stages of OA through a broad spectrum of anti-inflammatory and anti-catabolic effects.
Introduction
Osteoarthritis (OA) is a progressive, degenerative joint disease that affects more than 27 million Americans by compromising joint function and diminishing quality of life [1]. A hallmark of OA is the destruction of articular cartilage resulting in joint stiffness, swelling, pain, and loss of mobility [2]. OA is the leading cause of workplace disability and the source of $185.5 billion in annual medical care expenditures [3] The disease presents is a growing problem due to increased lifespans and increasingly obese Western populations [4]. Today, a cure for OA remains elusive [5], and there are no therapeutic agents with proven evidence that slow or halt the progression of OA [6]. As OA disease-modifying drugs are not yet available, the treatment of OA has been focused mostly managing symptoms such as pain and joint stiffness [7]. These treatments incorporate the use of pharmaceuticals such as nonsteroidal anti-inflammatory drugs (NSAIDs) [8]. However, while these commonly prescribed analgesics provide symptomatic relief, their long-term use has been associated with significant adverse gastrointestinal, renal, and cardiovascular side effects [7]. Furthermore, many existing analgesics have abuse liability and do not reduce pain in all treated individuals [9]. OA typically requires decades-long treatment, and as a result, there is an urgent need for alternative treatments that are effective and safe for long-term use.
Nutraceuticals are food or food-based products that provide physiological benefit or protection against chronic diseases. Nutraceuticals may have the ability to suppress many of the factors involved in the development and progression of OA including over-activated inflammation from pro-inflammatory cytokines (e.g. IL-6, TNF-α), and catabolic activity from proteolytic enzymes (e.g. MMP-13, ADAMTS-5) [10]. Importantly, as opposed to long-term pharmacological treatments which can result in adverse side effects, nutraceuticals are, by regulatory laws, safe for long-term use [11]. Drug N is a novel, proprietary nutraceutical formulation that was developed to target multiple factors involved in OA pathogenesis. The components of Drug N are recognized by the United States Food and Drug Administration (FDA) as generally recognized as safe (GRAS). Using a targeted screening approach, the components of Drug N were selected based on their ability to synergistically induce the expression of chondroprotective transcriptional regulator CITED2 (Cbp/p300 Interacting Transactivator with ED-rich tail 2) and reduce the expression of proteolytic enzymes and catabolic mediators involved in OA pathogenesis [12-14]. CITED2, in response to moderate mechanical loading, downregulates proteolytic enzymes ADAMTS5, MMP-1, and MMP-13 in vitro and in vivo [13, 14].
This study aims to test the therapeutic efficacy of Drug N in mitigating progression of OA in rats with posttraumatic OA induced by destabilization of the medial meniscus (DMM). Disease modification was assessed using Safranin O staining, Osteoarthritis Research Society International (OARSI) score, immunohistochemical staining of type II collagen and aggrecan cleavage epitopes, and gene expression analysis of pro-inflammatory cytokines and proteolytic enzymes.
Methods
Induction of osteoarthritis in rats and Drug N treatment
Destabilization of the medial meniscus (DMM) was performed by my mentor. DMM was established in Sprague Dawley rats (3-4 months old, male, n=6/group) by cleaving the medial meniscotibial ligament (MMTL) [15]. The joint capsule directly medial to the patellar tendon was incised and exposed using scissors. The patellar tendon was retracted and the MMTL was exposed by blunt dissection over the intercondylar area. Then, the MMTL was transected, causing destabilization of the medial meniscus. A sham operation was performed on the right hind limb using the same approach, but without MMTL transection. Afterwards, the joint capsule and skin were closed by suture [16]. Starting 4 weeks following surgery, rats were subjected to oral administrations of Drug N, ibuprofen, or placebo (cellulose) once daily for 4 weeks.
Safranin O Staining and OARSI score evaluation
Rats were sacrificed by my mentor 4 weeks following Drug N treatment. Hind limbs were fixed in formalin, decalcified in formic acid, embedded in paraffin wax, and sectioned for histology. Safranin O-fast green staining was used to visualize the integrity of the articular cartilage. OA severity was evaluated with 6-10 sections of the articular cartilage for each rat based on the Osteoarthritis Research Society International (OARSI) recommendations for histopathological assessment for OA [17]. In this system, the depth of cartilage degeneration is scored from 0 to 4 and the horizontal extent of cartilage involvement is scored from 0 to 4. The final score is product of the two factors (score range 0-16).
Immunohistochemical analysis
Immunohistochemistry was performed on deparaffinized and rehydrated sections. Primary antibodies against cleaved type II collagen (Col2-3/4, Ibex) and cleaved aggrecan (NITEGE, Ibex) were added and the sections were incubated overnight at 4°C. Subsequently, sections were incubated with anti-mouse or anti-rabbit secondary antibody (Biocare Medical) and visualized with DAB substrate (Vector Laboratories). Negative controls were prepared by staining with irrelevant isotype-matched antibodies (Biocare Medical). The immunofluorescent intensity for type II collagen or aggrecan cleavage epitopes was quantified with the RGB color system in Adobe Color Sampler [18]. The light intensity value was measured from six random locations within from the posterior to anterior direction of the femoral and tibial condyles, with three sections per rat [16].
Real-time PCR
The articular cartilage of naïve, sham and DMM rats treated with Drug N, ibuprofen, or placebo for 4 weeks was harvested. Total RNA was isolated from from formalin-fixed sections (PureLink® FFPE RNA Isolation Kit, ThermoFisher Scientific) according to manufacturer’s instructions. cDNA was synthesized using the iScript reverse transcription kit (Bio-Rad). Real-time polymerase chain reaction (PCR) was performed using SYBR Green Master Mix (Bio-Rad). Real-time PCR was performed in duplicate for each sample to determine relative gene expression using Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the housekeeping gene, with the 2-ΔΔCt method [19].
Statistical analysis
Results are expressed as mean ± SD. Comparative studies were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni’s correction for post hoc analysis, with GraphPad Prism software. P<0.05 was considered to be statistically significant.
Results
Drug N administration mitigates proteoglycan loss and cartilage degradation
Eight weeks after DMM surgery, placebo- and ibuprofen-treated rats exhibited moderate OA pathological changes in the articular cartilage, indicated by a loss of Safranin O staining, increase of cartilage erosion, and increase of cartilage fibrillation (Figure 1). In contrast, the Drug N-treated DMM rats exhibited significantly less OA pathological changes in the articular cartilage, compared to that treated with placebo and ibuprofen. Sham-operated and naïve rats exhibited no proteoglycan loss, cartilage erosion, or cartilage fibrillation.
To quantify the changes observed, a histologic grading system, Osteoarthritis Research Society International (OARSI) scores, was used (Figure 2). The placebo-treated DMM rats exhibited moderate proteoglycan loss and cartilage degradation, with an OARSI score of 5.50 ± 2.17. Similarly, ibuprofen-treated DMM rats exhibited moderate proteoglycan loss and cartilage degradation, with an OARSI score of 5.00 ± 1.90. In contrast, Drug N-treated DMM rats exhibited significantly reduced proteoglycan loss and cartilage degradation, with an OARSI score of 1.67 ± 1.03 (P<0.05). Sham-operated and naïve rats both exhibited no proteoglycan loss or cartilage degradation, with OARSI scores of 0±0.
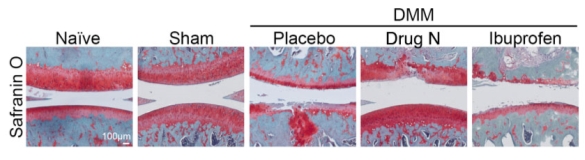
Figure 1. Drug N slows DMM-induced OA disease progression in rats by mitigating proteoglycan loss and cartilage degradation
Rats underwent sham surgery or destabilization of the medial meniscus (DMM). Four weeks after DMM surgery, rats were treated daily with Drug N, placebo, or ibuprofen for four weeks. Safranin O staining (red staining indicative of proteoglycans in the articular cartilage) revealed Drug N mitigated DMM-induced OA pathology, compared to that in the placebo and ibuprofen-treated animals (n=6/group). The blue color is indicative of the underlying subchondral bone.
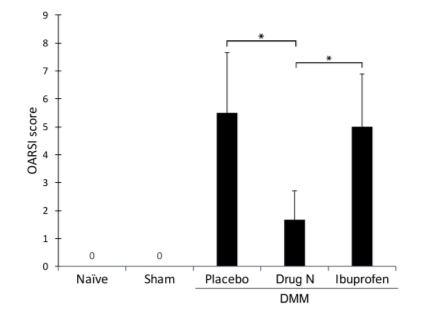
Figure 2. Osteoarthritis Research Society International (OARSI) scores indicate that Drug N treatment significantly reduces proteoglycan loss and cartilage degradation
The OARSI scores, a histologic grading system to assess cartilage integrity, are presented in the figure as mean ± standard deviation (*P<0.05 by t-test; n=6/group). 0=healthy cartilage; 16=maximum score of cartilage degradation.
Drug N reduces degradation of type II collagen and aggrecan in the articular cartilage matrix
The articular cartilage matrix is mainly degraded by the proteolytic enzymes of the MMP and ADAMTS families. MMP-13 is found to be the most potent enzyme in cleaving type II collagen, while ADAMTS5 is found to be the most potent enzyme in cleaving aggrecan [20]. Immunohistochemical staining revealed that Drug N treatment significantly reduced cleavage of type II collagen in DMM rats when compared to placebo- and ibuprofen-treated DMM rats (Figure 3). Quantification of the protein immunofluorescence using randomly selected areas of the articular cartilage revealed that the intensity of type II collagen cleavage epitopes of Drug N-treated DMM rats was increased 5.12-fold above naïve rats that had not undergone surgery or treatment (P<0.05). In contrast, cleaved type II collagen in placebo- and ibuprofen-treated rats increased 11.43- and 11.55-fold above naïve rats, respectively (P<0.05).
Immunohistochemical staining of aggrecan cleavage epitopes had similar results compared to that of cleaved type II collagen (Figure 4). Intensity of aggrecan cleavage epitopes in Drug N-treated DMM rats decreased to .987–fold below naïve rats (P>0.05). In contrast, immunofluorescent intensity of placebo- and ibuprofen treated rats increased 4.57- and 2.54-fold above naïve rats, respectively (P<0.05).
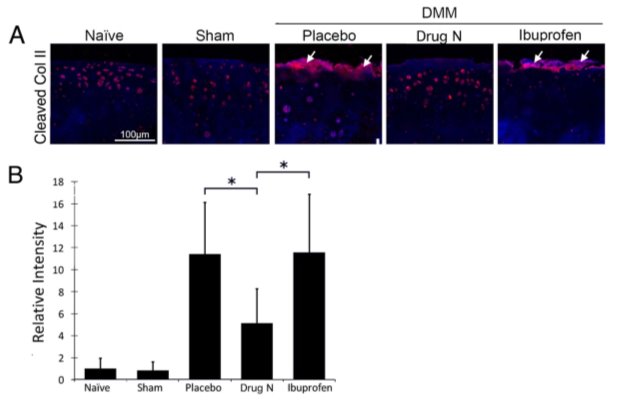
Figure 3. Drug N reduced cleavage of type II collagen, a major component of the articular cartilage matrix
(A) Immunofluorescent staining of type II collagen cleavage epitope (Col2-3/4 M) in the articular cartilage matrix of rats that underwent DMM and treated with placebo, Drug N, or ibuprofen at eight weeks following surgery. (B) Relative intensity of immunofluorescent staining. (*P<0.05 by t-test; n=6/group) White arrows highlight positive staining of cleaved type II collagen.
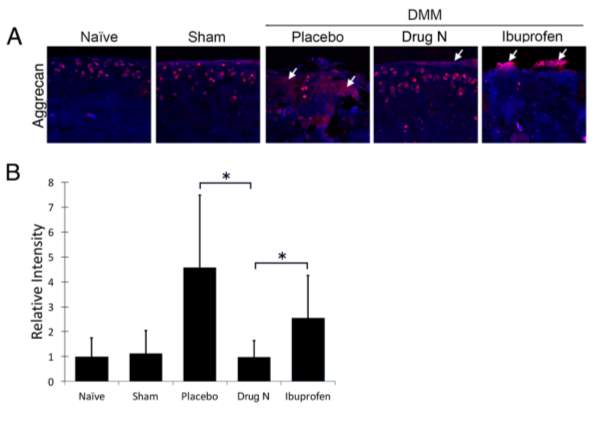
Figure 4. Drug N reduced cleavage of aggrecan, a major component of the articular cartilage matrix
(A) Immunofluorescent staining of aggrecan epitope (NITEGE, Ibex) in the articular cartilage matrix of rats that underwent DMM and treated with placebo, Drug N, or ibuprofen at eight weeks following surgery. (B) Relative intensity of immunofluorescent staining. (*P<0.05 by t-test; n=6/group) White arrows highlight positive staining of cleaved aggrecan.
Drug N administration results in a chondroprotective gene expression profile
To assess the potential therapeutic efficacy of Drug N and further understand the mechanisms underlying the effects of Drug N on cartilage integrity, the mRNA expression of genes that are closely related to cartilage homeostasis was examined using real-time PCR (Figure 5). Articular cartilage of rats treated with Drug N exhibited reduced mRNA levels of pro-inflammatory cytokines Il1b and Tnfa, and proteolytic enzymes Mmp13 and Adamts5 when compared to placebo and ibuprofen controls (P<0.05). The articular cartilage of rats treated with Drug N also exhibited increased expression levels of chondroprotective transcriptional regulator Cited2.
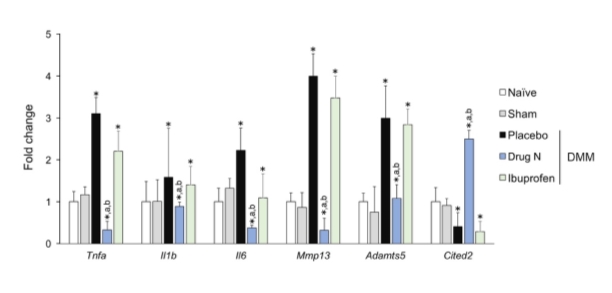
Figure 5. Drug N administration results in a chondroprotective gene expression profile
Drug N suppresses expression of pro-inflammatory cytokines Tnfa, Il1b, and Il6, and proteolytic enzymes Mmp13 and Adamts5 and induces expression of chondroprotective transcriptional regulator Cited2 in the articular cartilage. (*,a,b = P<0.05 vs naïve, placebo, and ibuprofen, respectively; n=6/group; one-way ANOVA with Bonferroni correction)
Discussion
Osteoarthritis (OA) is the most common form of arthritis, and presents a huge burden on individuals as well as the economy. The mainstay of current therapy include pharmacological interventions aimed at reducing pain symptoms and improving joint mobility. Currently, there are no FDA-approved therapeutic agents that can inhibit the disease progression of OA [21].
In this study, we provide the first evidence showing that oral administration of Drug N slows posttraumatic OA progression in a DMM rat model. We showed that Drug N, when given to rats with a moderate stage of OA, mitigates OA pathology through a broad spectrum of anti-inflammatory and anti-catabolic effects. Administration of Drug N resulted in reduced proteoglycan loss, less cartilage erosion and less cartilage fibrillation (Figure 1 and Figure 2), as well as improved preservation of the cartilage extracellular matrix, which is primarily composed of type II collagen and aggrecan (Figure 3 and Figure 4). These effects are corroborated by the reduced mRNA expression levels of genes associated with cartilage homeostasis, including inflammatory cytokines IL-1β, IL-6, and TNF-α, and catabolic mediators MMP-13 and ADAMTS-5. (Figure 5). Additionally, Drug N induces MMP- and ADAMTS-repressing transcriptional regulator CITED2 (Figure 5), which in previous studies has demonstrated its pivotal role in limiting OA progression in vitro [14] and in vivo [22].
The evidence provided in this study suggest that Drug N mitigates OA pathology, at least in part, by acting as an anti-inflammatory and anti-catabolic agent. These effects are extremely valuable because tissue damage is very difficult to reverse [23]. In particular, the extracellular matrix of human cartilage does not have the innate ability to regenerate because it is devoid of blood vessels, lymphatics, and nerves [24]. Thus, the preservation of articular cartilage are paramount to joint health.
Due to the nature of OA, therapeutic drugs are likely to require long-term administration in aging population. Additionally, studies have shown that the frequency of comorbidities is very high in OA patients, and OA is often comorbid with metabolic syndrome, diabetes, and depression [25, 26]. As such, patients with OA often take medication for coexistent diseases which may adversely affect other drugs. An advantage of nutraceutical treatments, including Drug N, are their favorable safety profiles. Drug N is composed of generally recognized as safe (GRAS, and are thus well tolerated for human consumption, likely without unintentional drug interactions.
Conclusions and Future Work
This study provides the first evidence that Drug N has significant efficacy in mitigating OA pathology, at least in part, by suppressing pro-inflammatory mediators and proteolytic enzymes, and inducing the expression of MMP- and ADAMTS-repressing transcriptional regulator CITED2. The therapeutic effects of Drug N observed in this study provide rationale for pursuing Drug N in clinical applications.
In addition to evaluating the efficacy of Drug N in a posttraumatic OA model, future studies may be conducted in order to validate the therapeutic efficacy of Drug N in other relevant OA models. Destabilization of the medial meniscus (DMM) was used in this study to surgically induce posttraumatic OA in rats. Eight weeks following the DMM surgery, rats exhibited altered joint biomechanics and pathological changes due to loss of meniscal function. These changes included cartilage erosion and fibrillation, subchondral bone thickening and formation of bone spurs, similar to symptoms observed in human OA [15]. In this study, the four-week time point following DMM surgery when the treatment began represented a moderate stage of OA, a clinical stage of disease representative of many patients seeking treatments [15]. However, there are some limitations to this acute rat trauma model. Due to the anatomical differences between rats and humans, the findings may not directly translate to humans. For example, progression of OA in humans is much slower than that in rats. Additionally, the experimental period only lasted until moderate stages of OA, so the therapeutic efficacy of Drug N in late-stage and severe OA is unknown. Future studies addressing the efficacy of Drug N on rats with late-stage OA, large animal models of OA, or other relevant models, such as spontaneous age-related OA, is of interest. If these studies validate the therapeutic efficacy of Drug N, it would provide sound rationale for advancing this potential drug toward clinical trials.
Lastly, future studies should measure the palliative effect of Drug N. Progressive joint failure accompanied by OA typically causes pain and physical disability, although the exact mechanisms are not well-understood [27]. In order for Drug N to be used as an alternative for current analgesics, it must also relieve pain symptoms and and preferably, improve joint mobility.
References
1. Lawrence, R.C., et al., Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum, 2008. 58(1): p. 26-35.
2. Musumeci, G., et al., Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci, 2015. 16(3): p. 6093-112.
3. Kotlarz, H., et al., Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum, 2009. 60(12): p. 3546-53.
4. Mitchell, N., et al., OBESITY: OVERVIEW OF AN EPIDEMIC. The Psychiatric clinics of North America, 2011. 34(4): p. 717-732.
5. Loeser, R.F., et al., Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum, 2012. 64(6): p. 1697-707.
6. Cheng, D.S. and C.J. Visco, Pharmaceutical therapy for osteoarthritis. PM R, 2012. 4(5 Suppl): p. S82-8.
7. Le Graverand-Gastineau, M.P., Disease modifying osteoarthritis drugs: facing development challenges and choosing molecular targets. Curr Drug Targets, 2010. 11(5): p. 528-35.
8. Jordan, K.M., et al., EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis, 2003. 62(12): p. 1145-55.
9. Okuse, K., Pain signalling pathways: from cytokines to ion channels. Int J Biochem Cell Biol, 2007. 39(3): p. 490-6.
10. Mentor, et al., Nutraceuticals: potential for chondroprotection and molecular targeting of osteoarthritis. Int J Mol Sci, 2013. 14(11): p. 23063-85.
11. Ameye, L.G. and W.S. Chee, Osteoarthritis and nutrition. From nutraceuticals to functional foods: a systematic review of the scientific evidence. Arthritis Res Ther, 2006. 8(4): p. R127.
12. Mentor, et al., C′-CEO slows post-traumatic OA progression and relieves OA-related pain. Osteoarthritis and Cartilage, 2015. 23: p. A337.
13. Mentor, et al., Physiological loading of joints prevents cartilage degradation through CITED2. FASEB J, 2011. 25(1): p. 182-91.
14. Yokota, H., M.B. Goldring, and Mentor, CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J Biol Chem, 2003. 278(47): p. 47275-80.
15. Iijima, H., et al., Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthritis and Cartilage, 2014. 22(7): p. 1036-1043.
16. Mentor, et al., Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model. Arthritis Res Ther, 2014. 16(6): p. 508.
17. Gerwin, N., et al., The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage, 2010. 18 Suppl 3: p. S24-34.
18. Nguyen, D., Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. 2013.
19. Schmittgen, T.D. and K.J. Livak, Analyzing real-time PCR data by the comparative CT method. Nat. Protocols, 2008. 3(6): p. 1101-1108.
20. Mort, J.S. and C.J. Billington, Articular cartilage and changes in arthritis: Matrix degradation. Arthritis Research, 2001. 3(6): p. 337-341.
21. Karsdal, M.A., et al., Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage, 2016. 24(12): p. 2013-2021.
22. Mentor, et al., The chondroprotective role of CITED2 in post-traumatic osteoarthritis. Osteoarthritis and Cartilage, 2013. 21: p. S304-S305.
23. Tuan, R.S., A.F. Chen, and B.A. Klatt, Cartilage regeneration. J Am Acad Orthop Surg, 2013. 21(5): p. 303-11.
24. Sophia Fox, A.J., A. Bedi, and S.A. Rodeo, The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health, 2009. 1(6): p. 461-468.
25. Leite, A.A., et al., Comorbidades em pacientes com osteoartrite: frequência e impacto na dor e na função física. Revista Brasileira de Reumatologia, 2011. 51: p. 118-123.
26. Kadam, U.T., K. Jordan, and P.R. Croft, Clinical comorbidity in patients with osteoarthritis: a case-control study of general practice consulters in England and Wales. Annals of the Rheumatic Diseases, 2004. 63(4): p. 408-414.
27. Hunter, D.J., J.J. McDougall, and F.J. Keefe, The symptoms of OA and the genesis of pain. Rheumatic diseases clinics of North America, 2008. 34(3): p. 623-643.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biomedical Science"
Biomedical Science focuses on how cells, organs and systems function in the human body and underpins much of modern medicine. Biomedical Science applies parts of natural and/or formal sciences to help develop advances in healthcare.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




