Seaweed as a Source of Nutraceuticals in Aquaculture
Info: 27695 words (111 pages) Dissertation
Published: 10th Dec 2019
Tagged: Biology
ABSTRACT
Vibrio species are the most widespread pathogen in the aquaculture and all over the aquatic ecosystem. It causes a high rate of mortality in fishes and shrimps. There are few pathogens like Vibrio parahaemolyticus, vibrio anguillarum and vibrio vulnificus which have been display broadly for causing harm to aquatic vertebrates and invertebrates life. Vibrios are the mostly opportunistic pathogen, none the less it is to a great extent primary and secondary pathogenic that affect most of the shrimps present all over the globe and also causes huge economic loss for aquaculture sector. This study deals with recognizing the pathogenicity of Vibrio parahaemolyticus species on Macrobrachium rosenbergii and building up a successful treatment against the disease using the natural products. The present study conducted revealed that seaweed extraction of bioactive compounds carried out using different solvents can be utilized for treating the vibrio infection. This can be utilized as an intramuscular medication or by adding into the shrimp’s food for treating the disease in aquaculture and we aim to develop nutraceutical to overcome harmful effects of antibiotics.
Key words: Vibrio parahaemolyticus, nutraceutical, shrimps.
INDEX
| Sr. No. | CONTENTS | Page No. |
| 1. | Introduction | |
| 1.1 | History of aquaculture. | 1 |
| 1.2 | Aquaculture in India.
1.2.1 Freshwater Aquaculture. 1.2.2 Brackish water aquaculture. 1.2.3 Mariculture |
1
1-3 3-4 4 |
| 1.3 | Problems while resulting aqua-farming. | 4-5 |
| 1.4 | Impact of antibiotic in aquaculture. | 5-6 |
| 1.5 | Seaweed as a bioactive compound. | 6 |
| 1.6 | Morphology of seaweed. | 6-7 |
| 1.7 | Biodiversity of seaweed in the Indian coast line. | 7-8 |
| 1.8 | Types of seaweeds.
1.8.1 Green algae. 1.8.2 Brown algae. 1.8.3 Red algae. |
8
8-9 9-10 10 |
| 1.9 | Antioxidant activity.
1.9.1 Characteristics of antioxidants. 1.9.2 Antioxidant mechanism. |
11-12
12 12-13 |
| 1.10 | Use of seaweeds.
1.10.1 Seaweed as a food. 1.10.2 Seaweed as a fertilizers and biogas production. 1.10.3 Seaweed as a animal feed. 1.10.4 Role of seaweeds in pharmaceuticals industries. |
13
13-14 14-15 15-16 16-17 |
| 1.11 | Scientific classification organism involved.
1.11.1 Shrimp (Mcrobracheium rosenbergii). 1.11.2 Seaweed (Sargassum tenerrimum). 1.11.3 Pathogen (Vibrio parahaemolyticus). |
17
17 18 18 |
| 2. | Literature review | |
| 2.1 | Seaweed. | 19-20 |
| 2.2 | Problems in aqua-farming and advantage of seaweed over it. | 21-22 |
| 2.3 | Seaweeds and its bioactivity. | 22-23 |
| 2.4 | Antioxidant activity of seaweeds. | 23-24 |
| 2.5 | Antioxidant and antibiotic activity of seaweed. | 24 |
| 2.6 | Vibriosis and other infections in aquaculture. | 24-27 |
| 2.7 | Seaweed for safety and quality attributes of foods. | 27 |
| 3. | Materials and methods | |
| 3.1 | Media and chemicals. | 28 |
| 3.2 | Glass water and plastic wares. | 28 |
| 3.3 | Collection and maintenance of seaweed. | 28-29 |
| 3.4 | Collection and maintenance experimental animals. | 29 |
| 3.5 | Bacterial culture. | 29 |
| 3.6 | Moisture contain of seaweed. | 29-30 |
| 3.7 | Preparation of seaweed extract. | 30 |
| 3.8 | Phytochemical analysis.
3.8.1 Test for tannin. 3.8.2 Test for flavonoid. 3.8.3 Test for phenols. 3.8.4 Test for coumarins. 3.8.5 Test for phlobatannin. 3.8.6 Test for Anthraquinones. 3.8.7 Test for saponin. 3.8.8 Test for alkaloid. 3.8.9 Test for carbohydrates. 3.8.10 Test for cardiac glycosides. 3.8.11 Test for quinines. 3.8.12 Test for triterpenoids. 3.8.13 Test for glycosides. 3.8.14 Test for proteins and aminoacides. 3.8.15 Test for steroids and phytosteroids. |
31
31 31 31 31 31 32 32 32 32 32 32 33 33 33 33 |
| 3.9 | Antioxidant activity.
3.9.1 Determination of total phenolic content. 3.9.2 Determination of total flavonoid content. 3.9.3Determination of Total Antioxidant Activity 3.9.4 Determination of free radicals scavenging by DPPH method 3.9.5 Hydrogen Peroxide Scavenging Capacity 3.9.6 Hydroxyl radical scavenging assay. 3.9.7 ABTS radical scavenging assay. |
33
33-34 34 34-35 35 35-36 36 37 |
| 3.10 | Characterization of active compounds.
3.10.1 GC-MS analysis for bioactive compounds. 3.10.2 HPLC analysis. 3.10.3 FT-IR analysis. 3.10.4 FT-RAMAN analysis. |
37
37 37-38 38 39 |
| 3.11 | In-vitro study of antibacterial activity of seaweed extract.
3.11.1 Preparation of experimental media. 3.11.2 Well diffusion method. 3.11.3 Preparation of seaweed extract disc. 3.11.4 Disc diffusion method. 3.11.5 Minimum inhibitory concentration (MIC). 3.11.6 Growth kinetic assay. |
39
39 39-40 40 40 40-41 41 |
| 3.12 | Experimental pathogenicity of bacterial culture.
3.12.1 Preparation of bacterial inoculum. 3.12.2 Infection by intramuscular injection. 3.12.3 Infection by immersion method. 3.12.4 Toxic bioassay for post larva. |
41
41-42 42 42 42-43 |
| 3.13 | Treatment.
3.13.1 Treatment using extract by intramuscular injection and immersion method. 3.13.2 Encapsulation of crude compound for feed treatment feed. 3.13.3 Treatment using the encapsulated seaweeds extract beads. 3.13.4 SME analysis. |
43
43 43 44 44 |
| 3.14 | Histopathological investigation. | 44-45 |
| 3.15 | Genotoxicity assay by comet analysis: | 45 |
| 4. | Result and dissuasion | |
| 4.1 | Moisture contain of seaweed. | 46 |
| 4.2 | Phytochemical analysis of seaweed. | 46-47 |
| 4.3 | Antioxidant activity.
4.3.1 Total phenolic content. 4.3.2 Total flavonoid content. 4.3.3 Total antioxidant activity. 4.3.4 DPPH radical scavenging. 4.3.5 Hydrogen peroxide scavenging capacity. 4.3.6 Hydroxyl radical scavenging assay. 4.3.7 ABTS radical scavenging assay. |
47
47-48 49 50 51 52 53 54 |
| 4.4 | Characterization of active compounds.
4.4.1 GC-MS analysis. 4.4.2 HPLC analysis. 4.4.3 FT-IR analysis. 4.4.4 FT-RAMAN analysis. |
55
55-58 58-60 61-64 65-66 |
| 4.5 | In-vitro study of antibacterial activity.
4.5.1 Well diffusion method. 4.5.2 Disc diffusion method. 4.5.3 Minimum inhibitory concentration. 4.5.4 Growth kinetic assay. |
67
67-68 68-69 69 70-71 |
| 4.6 | Experimental pathogenicity of bacterial culture.
4.6.1 Experimental pathogenicity intramuscular and immersion. 4.6.2 Post larvae toxicity. |
72
72 73 |
| 4.7 | Treatment and formulation of beads.
4.7.1 Treatment using intramuscular injection. 4.7.2 Encapsulation of crude and treatment using encapsulated beads. 4.7.3 SEM analysis of beads. |
74
74 75 76 |
| 4.8 | Histopathology investigation. | 76-77 |
| 4.9 | Comet assay. | 78 |
| 5. | Conclusion | 79 |
| 6. | References | 80-86 |
LIST OF FIGURES
| Figure No. | TITLE | Page No. |
| 1. | The structure of seaweed | 07 |
| 2. | Biodiversity of seaweeds | 08 |
| 3. | Green algae (Chorophyta) | 09 |
| 4. | Brown algae (Phaeophyta) | 10 |
| 5. | Red algae (Rhodophyt) | 10 |
| 6. | Shrimp | 17 |
| 7. | Seaweed | 18 |
| 8. | Pathogen | 18 |
| 9. | Total phenolic content graphical representation | 48 |
| 10. | Total flavonoid contain graphical representation | 49 |
| 11. | Total antioxidant activity graphical representation | 50 |
| 12. | Free radical scavenging by DPPH method graphical representation | 51 |
| 13. | Hydrogen peroxide scavenging activity graphical representation | 52 |
| 14. | Hydroxyl radical scavenging assay graphical representation | 53 |
| 15. | ABTS radical scavenging assay graphical representation | 54 |
| 16. | GC-MS of chloroform seaweeds extract | 55 |
| 17. | GC-MS of Dichloromethane seaweeds extract | 56 |
| 18. | GC-MS of ethanol seaweeds extract | 57 |
| 19. | GC-MS of petroleum ether seaweeds extract | 58 |
| 20. | HPLC of chloroform seaweeds extract | 59 |
| 21. | HPLC of dichloromethane seaweeds extract | 59 |
| 22. | HPLC of petroleum ether seaweeds extract | 60 |
| 23. | FT-IR of chloroform crude extract | 61 |
| 24. | FT-IR of chloroform purified extract | 61 |
| 25. | FT-IR of dichloromethane crude extract | 62 |
| 26. | FT-IR of dichloromethane purified extract | 62 |
| 27. | FT-IR of ethanol crude extract | 63 |
| 28. | FT-IR of ethanol purified extract | 63 |
| 29. | FT-IR of petroleum ether crude extract | 64 |
| 30. | FT-IR of petroleum ether purified extract | 64 |
| 31. | FT-Raman of dichloromethane crude extract | 65 |
| 32. | FT-Raman of dichloromethane purified extract | 65 |
| 33. | FT-Raman of ethanol crude extract | 66 |
| 34. | FT-Raman of ethanol purified extract | 66 |
| 35. | Well diffusion Chloroform extract | 67 |
| 36. | Well diffusion Dichloromethane extract | 67 |
| 37. | Well diffusion Ethanol extract | 67 |
| 38. | Well diffusion Petroleum ether extract | 67 |
| 39. | Well diffusion water extract | 68 |
| 40. | Control with Dichloromethane extract | 68 |
| 41. | Disc diffusion test | 69 |
| 42. | Controls | 69 |
| 43. | Growth kinetic of chloroform crude and purified extract graphical representation | 70 |
| 44. | Growth kinetic of dichloromethane crude and purified extract graphical representation | 70 |
| 45. | Growth kinetic of ethanol crude and purified extract | 71 |
| 46. | Growth kinetic of petroleum ether crude and purified extract | 71 |
| 47. | Control shrimp | 72 |
| 48. | Infected shrimp | 72 |
| 49. | post larvae toxicity assay graphical representation | 73 |
| 50. | Treated shrimp | 74 |
| 51. | Circular beads formation in 1N HCl | 75 |
| 52. | Dried beads | 75 |
| 53. | SEM analysis of encapsulated beads | 76 |
| 54. | Infected gills | 77 |
| 55. | Control gills | 77 |
| 56. | Infected Hepatopancreas | 77 |
| 57. | Control Hepatopancreas | 77 |
| 58. | Infected tissue | 77 |
| 59. | Control tissue | 77 |
| 60. | Infected cell by comet assay | 78 |
| 61. | Control cell by comet assay | 78 |
LIST OF TABLES
| Table No. | TITLE | Page No. |
| 1. | Shrimp classification | 17 |
| 2. | Seaweed classification | 18 |
| 3. | Pathogen classification | 18 |
| 4. | Results of moisture content | 46 |
| 5. | Result of phytochemical analysis | 46-47 |
| 6. | Total phenolic content | 48 |
| 7. | Total Flavonoid Content | 49 |
| 8. | Total antioxidant activity | 50 |
| 9. | Free radical scavenging by DPPH method | 51 |
| 10. | Hydrogen peroxide scavenging activity | 52 |
| 11. | Hydroxyl radical scavenging assay | 53 |
| 12. | ABTS radical scavenging assay | 54 |
| 13. | GC-MS compound of chloroform seaweeds extract | 56 |
| 14. | GC-MS compounds of Dichloromethane seaweeds extract | 57 |
| 15. | GC-MS compounds of ethanol seaweeds extract | 57 |
| 16. | GC-MS compounds of petroleum ether seaweeds extract | 58 |
| 17. | Results of HPLC | 60 |
| 18. | Minimum inhibitory concentration | 69 |
1. INTRODUCTION
- HISTORY OF AQUACULTURE:
Aquaculture market has globally raised significantly in past few decades, with a normal yearly development rate of 8.9 % since 1970, aquaculture is thought to be the quickest developing food creating division on the planet and records for around 36 % of the worldwide fish supply and just about 60% of the worldwide shrimp supply (FAO, 2014). As far as amount, cultivating of cyprinids dominate the aquaculture productions, with 25.4 million Tones, while the generation of salmonids and shellfish (shrimp and prawns) contributes with 3.2 and 4.3 million Tones individually (FAO, 2014). This rise brought up intensive fish culture practices where in the condition and stressors like grading, poor water quality, handling, transporting and over crowing are common. (FAO, 2014)
1.2 AQUACULTURE IN INDIA:
Aquaculture in India is divided in three different sectors depending upon the water bodies types and area. This type mainly are (i) Freshwater aquaculture (ii) Brackish water aquaculture and (iii) Mariculture
1.2.1 Freshwater aquaculture:
The advancement of freshwater aquaculture in the nation turned into a reality taking after the foundation of the Pond Culture Division at Cuttack in 1949 under the Central Inland Fisheries Research Institute (CIFRI), West Bengal. Noteworthy advancements occurred from there on standardization of induced breeding methods and the improvement of hatchery facility frameworks and composite carp culture of the three Indian major carps and three exotic carps, including silver and grass carp, shaping the reason for carp polyculture frameworks. Freshwater aquaculture movement is noticeable in the eastern part of the nation, especially in the states like West Bengal, Orissa and Andhra Pradesh with new areas coming under culture in the states of Punjab, Haryana, Assam and Tripura (Anjani, Joshi et al., 2003).
India has the fourth biggest market in reference to the production of fish and shrimp and is assuming a vital part in worldwide fisheries. Furthermore, with production more than 3.1 million metric tons, the nation possesses second position on the planet in the inland fisheries segment. Over the most recent five decades, Indian fisheries have made incredible steps, with the yearly production expanding from 0.75 million tons of fish and shellfish in 1950 to around 6.1 million tons in the year 2002, an expansion of more than eight folds. The share of inland fisheries segment, which was 29% in 1950-51, has gone up to more than 50% at present. While capture fisheries have exclusively contributed production from the marine sector, aquaculture contributes from the inland fisheries area has been huge in recent years. The production from capture fisheries over the most recent two decades has developed by just 72% i.e., from 2.08 million tons in 1980 to 3.59 million tons in 2000, yet the aquaculture segment has demonstrated a development of 46.8% in a similar period, i.e., 0.37 million tons in 1980 to 2.1 million tons in 2000. India has likewise risen as one of the significant nations in international export in aquaculture, recording a growth peak the year 2000-2001 with income of Rs. 5,957 cores (US$ 1.25 billion). In some case, there has been a decrease of 7.56% during 2001-2002 because of financial steep and recession decrease in costs of the tiger prawns in the global market (Ayyappan et al., 1999).
Indian aquaculture has indicated essentially higher development rates than those of capture fisheries in the most recent decade, with the amount expanding from 1.01 million tons in 1990 to 2.10 million tons in 2000. Freshwater aquaculture has kept on having a maximum share in the aquaculture department, with a contribution of more than 95% in quantity. It is just the three Indian major carps, which share as much as 1.6 million tons of the production. Then again, shrimp are the principle segment of salty water aquaculture department with the production crossing lakh tons mark, as of recent. Freshwater aquaculture in India has made long walks improvement as of recent with a development trend similar that of the world. With a yearly development rate of more than 6% during the most recent decade, the sector possesses higher growth rates than other food producing sectors.
Despite the fact that the nation has countless cultivable carp species, it is just the three Indian major carps; (catla), rohu (Labeo rohita) and mirgal (Cirrhinus mrigala), that contribute a lion’s share to a generation of 0.546, 0.567 and 0.517 million tons, separately, recorded during the year 2000. Logically viewed over the most recent five decades have prompted the advancement of a large group of carp culture technologies with differed production possibilities relying upon the sort and level of input. Further, other produce like catfishes, freshwater prawns and molluscs for pearl culture have additionally been brought into the way of aquaculture frameworks. Moreover, a scope of other non-conventional culture syatem, similar to sewage-fed fish culture, integrated farming; cage and pen culture and running water fish culture have made freshwater aquaculture a growing activity across the country (Gopakumar et al., 1999).
1.2.2 Brackish water aquaculture:
Brackish water farming in India is a well-established system restricted for the most part to the berries (manmade impoundments in coastal wetlands) of West Bengal and pokkali (salt resistant deep-water paddy) fields along the Kerala coast. It is being assessed that 1.2 million hectares of potential saline water range accessible in India are suitable for aquaculture activity. Furthermore, around 8.5 million hectares of salt influenced regions are likewise accessible, of which around 2.6 million hectares could be only used for aquaculture because of the unacceptability of these resource for other agribusiness based activities. The farming of shrimp is largely dependent on small holdings of less than 2 hectares, these farms accounting for over 90 percent of the total area utilized for shrimp culture, while large holdings of over 10 ha account for only 1.54 percent of the total. A number of the ranch property situated in Kerala and West Bengal have a place with the conventional system of shrimp cultivating (Rao et al., 2001).
Various Government programmers have been initiated to provide support to the shrimp farming sector augmented its development. The MPEDA (Marine Products Export Development Authority) has constantly focused to boost the shrimp-farming sector. Farmed shrimp production increased from 40,000 tons in 1991–1992 to 115,000 tons in 2002–2003. Out of the total area of 0.152 million ha presently being utilized for shrimp farming in the country, Andhra Pradesh alone accounts for 47 percent of the area and contributes 50 percent of the total production. India is commercial producer of brackish water finfish is almost in continues process and various monoculture as well as polyculture of pearl-spot, milkfish , sand whiting and mullets have shown their potential for farming.
The brackish water aquaculture division is for the most part upheld by shrimp production and goliath tiger prawn (Penaeus monodon) production, which are in charge of the heft of production taken after by the Indian white prawn, P. indicus. In spite of the fact that India has a few other potential types of finfish and shellfish, production of these is still low. In seawater, the major farmed species are the green mussel (Perna viridis), brown mussel (Perna indica), Indian backwater shellfish (Crassostrea madrasensis), Japanese pearl clam (Pinctada fucata) and seaweeds species like Gracilaria edulis.
1.2.3 Mariculture:
Mariculture is the term utilized for the farming of marine living creature in seawater, normally in sheltered in coastal water (Neori et al., 2004). Specifically, the cultivating of marine fish is an example of mariculture, thus additionally the farming of marine crustaceans, (for example, shrimps), molluscs, (for example, shellfish) and seaweeds. mariculture is a more efficient user of primary productivity than the farming of livestock.
The first attempt at mariculture in India was made at the Mandapam centre of CMFRI (Central Marine Fisheries Research Institute) in 1958–1959 with the culture of milkfish (chanos). In the course of the most recent three decades, CMFRI has created different advance technologies for various species including shellfish, mussels and mollusks among sedentary species, and also for shrimps and finfish (FAO . 2005 . Aquaculture generation, 2003).
1.3 PROBLEMS WHILE PERSUING AQUA-FARMING
It is always been demonstrated around the globe that farmed fishes are highly susceptible to disease agents because of stressors posed due to intensive rearing. High rate of mortality are caused due to bacterial infection in aquaculture organisms as well as human population. Most of infection are caused due to the primary pathogens of fishes, which causes systemic infection in fishes leading to cause of disease and death. The considerable economic loss is seen in development of aquaculture due to pathogen of vibrio species
.[3]Illnesses, either irresistible or non-irresistible, are critical constraining variables that influence the production volume and subsequently the production cost. In 2006, for a worldwide production of 1.6 million Tons of salmon, the cost for ocean lice medication was evaluated at 305 million € (Costello, 2009). It was estimated that in Norway, the top salmonid producer on the planet, the cost of ocean lice control is around 0.19 € kg-1 of salmon (Costello, 2009). Moreover, it was assessed that in 2010, more than 77 million USD were spent in Norway on fish illnesses administration, including the usage of enactment and support to observation and control programs (The Fish Site, 2010)
1.4 IMPACT OF ANTIBIOTIC IN AQUACULTURE:
Nowadays, use of antibiotic in aquaculture has significantly raised due to heavy infection because of indiscriminate use of antibiotic the pathogenic bacteria are becoming drug resistant is common issue. This issue has become greater problem in treatment against disease caused by this drug resistant pathogenic bacteria. Diminished effectiveness and resistance of pathogen to antibiotics have required the improvement of new options
.5Among these new options, the utilization of different common items that get from various living organisms, for example, animal (e.g. chitozan), plant (e.g. essential oil) and kelp has got a considerable measure of attention around the globe (Romero et al., 2012). In addition, the cost of the medications is high and furthermore they cause antagonistic impact on the host, which incorporate extreme hypersensitivity and exhaustion of advantageous microorganisms in the gut. (Smit, 2004) Because of the flare-up of irresistible illnesses brought on by various pathogenic microscopic organisms and the advancement of antibiotic resistance, the pharmaceutical organizations and the analysts are presently in search new antibacterial agents (Smit, 2004). Anti-bacterial treatment can influence target not over only on pathogen but also has its influence over commensal inhabitant of the human host. The degree of the effect on non-target microbial population relies on upon the specific anti-bacterial used, its method of activity and the level of resistance in the group. Once in a while an unevenness in the commensal gut microbiota can happen because of administration of anti-biotic can bring about intestinal issues, for example, antibiotic-associated diarrhea (McFarland, 1998). As of late, it has been demonstrated that even short term anti-biotic administration can prompt to stabilization of resistant bacterial populaces in the human digestive tract that continues for a considerable length of times (Jakobsson et al., 2010; Jernberg et al., 2007; Lo¨fmark et al., 2006)..
1.5 SEAWEED AS BIOACTIVE COMPOUND:
Bioactive characteristic items are generally and highly found in the plant kingdom, and the extract from various plants and in addition green, brown and red algae can be utilized as naturally occurring bioactive products. Marine algae are the representative of an inexhaustible supply of crude materials utilized as a part of pharmaceutical, food industries, medicine and beautifiers. Marine algae fill in as a vital wellspring of bioactive substances. Marine algae fill in as an import Special consideration has been paid to antibacterial exercises identified with marine algae against a few pathogens. The active constitution and extract of different marine algae have been studied to screen for antimicrobial action showed against various Gram-negative and Gram-positive microbial population. The antibacterial compound got from the marine algae comprise of a different gathering of synthetic compounds. (Smit, 2004)
1.6 MORPHOLOGY OF SEAWEED:
Seaweeds do not have strong support structures, Rather than roots seaweeds have holdfasts, which connect them to the ocean bottom. A holdfast is not usually for water and supplement uptake, but rather is required as an anchor.The stem or stalk of kelp is known as stipe. The stipe’s function is to support the whole plant. The stipe structure differs among different seaweeds and it can be gas-filled, solid, firm, flexiable, gas-filled, short, long (20 meters), or totally absent (Druehl, 2000).
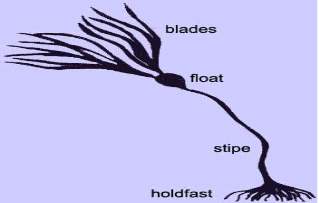
Fig 1: The structure of seaweed
Seaweeds leaves are known as blades. The fundamental function of the blades is to give a huge surface to it for the absorbance of light. In a few species the blade additionally gives support to the reproductive structures. A few seaweeds have just a single blade, which might be separated, while other different species have various blades (Harbo, 1999).
Numerous kelp has empty, gas-filled structures called pneumatocysts or floats. These assist in keeping the photosynthetic structures floating so that energy can be absorbed from the sun (Turner, 2003).
Absorption of the nutrients happens directly through the thallus walls. The thallus might be just a simple column of cells or filament of call, a flattened membrane film of one or several few layers of cells or a complex arrangement of organs including regenerative organs and stipe holding photosynthetic frond (Tudge, 2000).
1.7 BIODIVERSITY OF SEAWEEDS IN THE INDIAN COAST LINE:
India is presented with a long coastline it is about 7,516 Km and sizeable (2.5 million SQ km) Exclusive Economic Zone (EEZ) which is around two third of the territory range. The seaweed or algae flora of India is exceedingly enhanced and involves for the most part of tropical species.(Jha et al. 2009)
As indicated by Untawale et al. (1983) Indian coastline consist of 844 types of marine algae having a place with 215 genera and 64 families. Bay of Mannar took first place as far as density and diversity of species in concerned (302 species), Gulf of Kutch takes second place (202 species). The biodiversity of kelp in Gulf of Mannar is because of the substantial degree of coral reefs, which give an appropriate substrate to its development (Kannan et al., 2006).
Diversity of seaweeds is abundant in Gulf of kutch, Gulf of Mannar, Andaman and Nicobar Islands and Lakswadeep. (Gopinath, 2014)
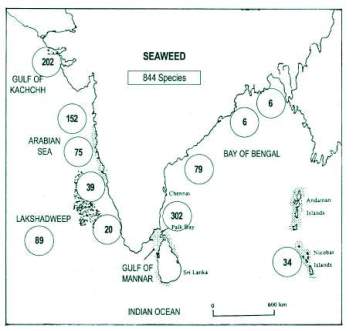
Fig 2: Biodiversity of seaweeds
1.8 TYPES OF SEAWEEDS:
Three main groups of seaweed are seen and are characterised by their pigments that assimilate light of specific wavelengths and give them their characteristic shades of green, browm or red (Collins, 2001).
1.8.1 Green algae:
More than 4,000 species of different green algae are found in nature. They are found in different habitats like freshwater or marine water, and many others thrive in moist soil. They come in three forms: unicellular, colonial or multicellular. The green algae are also sometimes termed as Chlorophyta. The Chlorophyta are genuinely green with no pigment that masks the chlorophyll. The green algae are highly diverged and also found in various forms that are small little microscopic single free-swimming cell to bushy plants and large membranous. (Davies, 2002).Examples of green algae: sea lettuce (Ulva sp.), which is commonly found in tide pools, Codium sp., one species of which is commonly called “dead man’s fingers.”

Fig 3: Green algae (Chorophyta)
1.8.2 Brown algae:
Brown algae are multicellular and are found in a variety of distinctive physical structures consisting of filaments and crusts. Like other photosynthetic life forms, Brown algae also consist of green chlorophyll pigment. They additionally have gold and brown pigment, which mask the green shade of chlorophyll. The prominent pigment found in this algae called fucoxanthin (Bartle, 2005). Brown algae are the largest type of all algae. They are placed in the phylum Phaeophyta, which signifies “dusty plants.” These algae are yellow-brown or brown colored and found in mild or cold waters. Brown algae ordinarily have root-like structures known as “holdfast” that helps to stay adhere to any rough surface.
Example of brown algae: kelp, Sargassum, rockweed (Fucus).

Fig 4: Brown algae (Phaeophyta)
1.8.3 Red algae:
The red algae (Rhodophyta) not only has chlorophyll contain but also contain pigments like phycocyanin and phycoerythrin which are responsible for red colouration. Red algae are found in various different forms of physical structures, including simple basic and branched filaments (Harbo, 1999). More than 6000 species are found in nature of red algae. These algae contain its brilliant colour due to the two additional pigments that it contains. These algae absorb blue wavelength light and can grow at a greater depth than green algae and brown algae. Coralline algae, a group of red algae, are the important forms of algae which develop into coral reefs. An example of red algae: Irish moss, coralline algae, dulse (Palmaria palmata).

Fig 5: Red algae (Rhodophyt)
1.9 ANTIOXIDANT ACTIVITY:
Antioxidants are the agents which scavenge the free radicals and protects from the harm brought on by them. They can extraordinarily diminish the damage because of oxidants by neutralizing the free radicals before they can assault the cells and avert harm to lipids, proteins, compounds, sugars and DNA (Fang et al., 2002). Antioxidants agents can be ordered into two noteworthy classes i.e., enzymatic and non-enzymatic. The enzymatic antioxidants are created endogenously and incorporate superoxide dismutase, catalase, and glutathione peroxidase. The non-enzymatic antioxidants incorporate tocopherols, carotenoids, ascorbic corrosive, flavonoids and tannins which are acquired from natural sources (Lee et al., 2004). An extensive variety of antioxidants from both natural sources and synthetic sources has been put forth for use in the treatment of different human illnesses (Cuzzocrea et al., 2001). There are antioxidants agent synthetic origin, for example, butylated hydroxytoluene, butylated hydroxyanisole and tertiary butyl hydroquinone which are generally utilized as a part of processed food product. It has been demonstrated that these mixes have indicated harmful impacts like liver harm and mutagenesis (Grice, 1986; Wichi, 1988). Flavonoids and other phenolic mixes of natural origin have been accounted for scavenging of free radicals (Formica and Regelson, 1995; Rice-Evans et al., 1997). Subsequently, these days look for the regular antioxidants agent source is increasing its significance.
Antioxidants are generally utilized as a part of dietary supplements and have been explored for the prevention of various disease, for example, cancer, coronary illness and even height ailment. Although in some study reviews it’s recommended that antioxidants supplements may advance wellbeing of health, later vast clinical trials with a set number of antioxidants identified no advantage and even proposed that abundance supplementation with certain putative antioxidants might be unsafe. Antioxidants likewise have numerous uses in many industries, for example, it can be used additives in food for preservation and beauty care products and to prevent rubber and gasoline from degrading.
These days antioxidant activity is primarily focused because of right now developing interest from the pharmaceutical business where there is enthusiasm for anti-aging and anti-carcinogenic nature of its bioactive mixes, which have medical advantages. Additionally, antioxidant and materials having antioxidant activity are utilized broadly for the development of nourishment steadiness. With the focus is being moved towards discovering choices for naturally engineered nourishment ingredients, naturally occurring substances having anti-oxidative properties are in tremendous demand around the world. Naturally occurring antioxidant agents are viewed as safe for use in drug, dietary supplements, nutraceuticals, the cosmetic world with the target of enhancing the wellbeing of people, decreasing the impacts of unsafe illnesses and other more extensive parts is to enhance immune system function using such properties.
Phenolic compounds, for example, flavonoids, phenolic acids, and tannins are thought to be significant supporters of the antioxidant property of various naturally occurring materials. Phenolic or polyphenols have got significant consideration on account of their physiological capacities, including antimutagenic anti-oxidants and antitumor action. Phenolic compounds are usually had access from eatable green, brown and red seaweeds, whose antioxidant activity have been connected to their phenolic content (Bartolomeu Souza et al., 2011).
1.9.1 Characteristics of Antioxidants:
The major antioxidant as of now utilized as a part of nourishments is monohydroxy or polyhydroxy phenol compounds with different ring substitutions. These phenolic compounds have low activation energy to donate hydrogen. Other free radical are not initiated due to stabilization of delocalization of radical electrons.
The resulting antioxidant free radical is not subject to rapid oxidation due to its stability. The antioxidant free radicals can also react with lipid free radicals to form stable complex compounds.
1.9.2 Antioxidant mechanism:
Hydrogen donation to free radicals by antioxidants.
Formation of a complex between the lipid radical and the antioxidant radical (free radical acceptor).
Reaction of antioxidants with radicals:
 R· + AH RH + A·
R· + AH RH + A·
 RO· + AH ROH + A·
RO· + AH ROH + A·
 ROO· + AH ROOH + A·
ROO· + AH ROOH + A·
 R· + A· RA
R· + A· RA
 RO· + A· ROA
RO· + A· ROA
 ROO· + A· ROOA
ROO· + A· ROOA
 Antioxidant + O2 Oxidized Antioxidant
Antioxidant + O2 Oxidized Antioxidant
1.10 USES OF SEAWEEDS
1.10.1 Seaweed as a foods:
Red micro-algae (Gracilaria spp.) are utilized as a food in Hawaii. Species usually marketed are G. parvispora, G. coronopifolia, G. parvispora and G. salicornia this seaweeds have a short postharvest life of around 4 days (Paul and Chen, 2008). This seaweeds are a rich wellspring of phytochemicals having antioxidant properties and antimicrobial properties. The fibers and minerals present in seaweeds help in enhancing the mineral substance diminishes the salt amount. The including seaweeds and product helps in food preservation. (Gupta and Abu-Ghannam, 2011). Eatable seaweed has different bioactive properties with potential medical advantages and their utilization as functional ingredients has bought up new prospects in food industries, meat item formulations included. Seaweeds fundamentally contain high extents of polysaccharides and also different other conceivably valuable compounds, for example, great quality protein and basic unsaturated fats, especially long-chain n-3 polyunsaturated fatty acids (PUFAs). Alginates are the most ionic polysaccharides known in brown seaweeds (Fernández-Martín et al., 2009). In Some seaweeds polysaccharides are utilized by the food industries as a part of business and used as texture modifiers due to their gelling properties and high viscosity. In Asia, seaweeds is been utilized for quite a long time period in plates of mixed greens, soups, and as low-calorie diet food. The dietary fiber which have about 25-75 % of the dry weight of marine green algae and has represented to their major component, is principally a soluble fibre.(Jiménez-Escrig and Sánchez-Muniz, 2000). In some part around globe, miyeok (Undaria pinnatifida) is served in salad, soup, and side dishes.
1.10.2 Seaweeds as fertilizers and biogas productions:
Seaweeds are utilized as a compost which is appropriate for use in natural farming (López-Mosquera et al., 2011). Energy-rich compounds like methane can be harnessed from kelp deposits by anaerobic processing. However, the heavy metal substance in the seaweeds and its digestates limits their utilization as composts. The effective use of seaweeds is for biogas creation, and the incomplete substantial metals mobilization to empower the metal evacuation for enhanced compost quality (Nkemka and Murto, 2012). The seaweed (Chondracanthus squarrulosus) was cultured under semi-controlled conditions to assess development (biomass creation) with farming manures (ammonium nitrate, ammonium sulfate and urea) versus analytical grade inorganic salts; sodium nitrate (systematic review) and seawater were utilized as controls (Pacheco-Ruiz et al., 2004).
There is a long history of coastal people utilizing kelp, particularly the brown seaweeds, to fertilize the land near coastal areas. Wet ocean growth is heavy to carry due to water content so it was not for the most part in extremely far inland, in spite of the fact that the wet seaweeds are heavy to carry the west bank of Ireland eagerness was to such an extent that it was transported far long kilometers from the shore. Generally drift seaweeds or shoreline washed kelp is gathered, in spite of the fact that in Scotland ranchers now and then cut Ascophyllum exposed at low tide. In Cornwall (United Kingdom), the practice was to form a mixture of the sand with seaweeds, let it spoil and after that dig it in the land. For over a couple of hundred kilometers of the coastline around Brittany (France), the shoreline cast, dark colored kelp is consistently gathered by ranchers and utilized on fields up to a kilometer inland. Comparative practices can be seen in some nations around the globe. for example in a more tropical atmosphere like the Philippines, extensive amounts of Sargassum have been gathered, utilized wet locally, additionally sun dried and transported to different regions. Some portion of this algal mass has been treated the soil and afterward utilized as a part of trials for developing tomato plants in different sorts of soil. In all cases, the expansion of the manure expanded water holding limit and plant development, so treating the soil at the same time tackled natural contamination issues and created a valuable natural compost.
Seaweed meal is dried, processed kelp, and again it is normally in view of the brown seaweeds since they are the most promptly accessible in huge amounts. species of Ascophyllum, Ecklonia and Fucus are the regular ones. They are sold as soil additives substance and acts as both compost and soil conditioner. They have an appropriate substance of nitrogen and potassium however are much lower in phosphorus than animal excreta composts and the typical N:P:K proportions in compound manures. A lot of insoluble starches in brown seaweeds plays role in as soil conditioners (enhance air circulation and soil structure, particularly in mud soils) and has moisture holding properties to a great level. Their adequacy as composts is likewise once in a while credited as trace elements they contain, however, the real commitment they make is little contrasted with typical plant requirment.
1.10.3 Seaweeds as animal feed:
For quite long time, animals for example, sheep, cows and hourses that lived near coastal ranges have eaten seaweeds, particularly in those European nations where huge brown seaweeds were washed shorewards. Today the accessibility of kelp for living creature has been expanded with the production of seaweeds meal: dried seaweed that has processed to a fine powder. Norway is among the first in the list of makers of seaweeds, utilizing Ascophyllum nodosum, a kelp that develops in the eulittoral zone so it can be cut and gathered when exposed at low tide.
The seaweeds utilized for meal must be properly processed and cut, as drift seaweeds is low in minerals and typically prone noticeably contamination and becoming infected with microbial forms like mold. The wet seaweeds is gone through milling hammer with dynamically smaller screens to diminish it to fine particles. These are gone through a drum dryer beginning at 700-800°C and leaving at close to 70°C. It has to contain a water content level of around 15 percent. It is processed and put away in fixed airtight packs to prevent it from getting up moist if presented to air. It can be stored away for about a year.
In fish cultivating, fish feed more often comprises of meat waste and fish squander blended with added substances containing additional supplements, all makes up together in a raw mass. When dropped into the fish lakes or ponds and tanks it must hold together and should not be soluble in water or disintegrate in the water. A binders are necessary, some of the time a specialized grade of alginate is utilized. It has additionally been utilized to bind formulated meals for shrimp and abalone. Be that as it may, less expensive still is the utilization of finely ground kelp produced using darker brown seaweeds; the alginate in the seaweeds actively acts as gelling agent or binders. The binder might be a critical extent of the cost of the feed so seaweed meal is a greatly improved decision.
There is additionally a business opportunity for seaweeds as feed for shrimps. In Australia, the brown seaweeds (Macrocystis pyrifera) and the red ocean growth (Gracilaria edulis) have been utilized as fish feeds. In South Africa, Porphyra is in great demand for fish and shrimp nourishment supplement and proposals have been made for the administration of the wild populace of the seaweeds. Pacific dulse (Palmaria mollis) has been observed to be a significant meal for the red abalone, Haliotis rufescens, and improvement of land-based development has been attempted with a view to creating a business using the seaweeds. The green seaweed, Ulva lactuca, has been given to Haliotis tuberculata and H. plate. Bolstering and various other fishes and shrimps trials demonstrated that fishes and shrimps development is significantly enhanced by a high protein substance, and this is accomplished by refining the seaweeds with large amounts of ammonia present. A significant part of the work on ocean growth and abalone has been published in the journals Aquaculture and Journal of Shellfish Research.
1.10.4 Role of seaweeds in Pharmaceutical industries:
In the last three decades the discovery of metabolites with biological activities from macroalgae has increased significantly. However, despite the intense research effort by academic and corporate institutions, very few products with real potential have been identified or developed. Based on Silverplatter medicine and Aquatic Biology, Aquaculture & Fisheries Resources databases, the literature was searched for natural products from marine macroalgae in the Rhodophyta, Phaeophyta and Chlorophyta with biological and pharmacological activity. Substances that currently receive most attention from pharmaceutical companies for use in drug development, or from researchers in the field of medicine-related research include: sulphated polysaccharides as antiviral substances, halogenated furanones from Delisea pulchra as antifouling compounds, and kahalalide F from a species of Bryopsis as a possible treatment of lung cancer, tumours and AIDS. Other substances such as macroalgal lectins, fucoidans, kainoids and aplysiatoxins are routinely used in biomedical research and a multitude of other substances have known biological activities. The potential pharmaceutical, medicinal and research applications of these compounds are discussed in many research works and also used in pharma industries. (Smit, 2004)
1.11 SCIENTIFIC CLASSIFICATION ORGANISMS INVOLVED:
1.11.1 Shrimp (Macrobrachium rosenbergii)
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Malacostraca |
| Order: | Decapoda |
| Family: | Palaemonidae |
| Genus: | Macrobrachium |
| Species: | M. rosenbergii |
Table 1: Shrimpclassification Fig 6: Shrimp
1.11.2 Seaweed (Sargassum tenerrimum)
| Domain: | Eukaryota |
| Superphylum: | Heterokonta |
| Class: | Phaeophyceae |
| Order: | Fucales |
| Family: | Sargassaceae |
| Genus: | Sargassum |
| Species: | S. tenerrimum |

Table 2: Seaweed classification Fig 7: Seaweed
1.11.3 Pathogen (Vibiro parahaemolyticus)
| Kingdom: | Bacteria |
| Phylum: | Proteobacteria |
| Class: | Gamma Proteobacteria |
| Order: | Vibrionales |
| Family: | Vibrionaceae |
| Genus: | Vibrio |
| Species: | V. parahaemolyticus |
Table 3: Pathogen classification Fig 8: Pathogen
OBJECTIVE:
- Collection of seaweeds
- Extraction of bioactive compound by Maceration
- Antioxidant activity
- Comparison of antioxidant activity seaweed in different solvent
- Determination of best seaweeds extraction for treatment
2. LITERATURE REVIEW
2.1 SEAWEEDS:
Algae are a huge and very diverse group of chlorophyll-bearing, simple, photosynthetic, thalloid living beings to a great extent sea-growing with no separation of roots, stems and leaves like structure, having been place in the division Thallophyta (NCERT 2008). Seaweeds are separated into three classes: Chlorophyceae, Phaeophyceae and Rhodophyceae (Gamal 2010). Algae are mostly found in the aquatic environment in both marine water and fresh water. Algal living spaces include terrestrial, for example, wet rocks and soggy soil and sub elevated, similar to tree coverings; they can prosper at low temperatures. Some algae play a role like symbiotic association in nature with organisms like Lichens and animals e.g. sloth bear (NCERT 2008). Algae might be free floating above the surface of the water, for example, Chlamydomonas or they can be connected to substratum e.g. Ulothrix and Sargassum. Algae have diverse shape and size from unicellular (e.g. Chlamydomonas) to multi-cell (e.g. Laminaria), pilgrim (e.g. Volvox) to filamentous structures (e.g. Ulothrix) and tiny to gigantic plant bodies (NCERT 2008). Algae are secured by mucilage similarly as other aquatic plants. Algae do not contain vascular tissues and mechanical tissues in light of the fact that being aquatic, they don’t require water conduction and buoyancy keep them upright in water (NCERT 2008). They reproduce by different means which can be vegetative, agamic and sexual. Vegetative propagation happens through fragmentation. A sexual proliferation in algae is expert by spores called zoospores, which are of two sorts: mitospores and meiospores. Sexual reproduction includes isogamy, anisogamy and oogamy (NCERT 2008).
Algae can be utilized as food and nourishment supplement for people and they are a primary producer in the food chain for aquatic life. Some marine algae also produce hydrocolloids (water holdind substances, for example, algin (from Laminaria), carrageenan (from Chondrus) and agar (from Gracilaria and Gelidium) which can be utilized as a part of producing a variety of business items. Algae can carry out photosynthesis, fix carbon dioxide and they produce the very high amount of oxygen. Seaweeds are morphologically and physiologically distinct from both land plants and freshwater algae. They contain a huge measure of polysaccharides in their cell wall. Some of these seaweeds contain compounds like carrageenans; alginates and agar which are broadly utilized by cosmetic, food and pharma industries.
Presence of metabolites such as fatty acids, steroids, carotenoids, lectin and mycosporine like amino acids, halogenated compounds ploypeptides and toxins as well as other sulphated polysaccharides make these organisms an economically important product (Cardozo et.al. 2007). Seaweeds are the excellent source of bioactive compounds such as carotenoids, dietary fibre, protein, essential fatty acids, vitamins and minerals (Bhaskar et.al, 2005), (Miyashita et.al, 2005). Marine macro algae are usually classified based on their characteristic phyto pigment such as: Rhodophyta (Red Algae), Phaeophyta (Brown Algae), and Chlorophyta (Green Algae).
In recent years, there have been many reports of macro algae derived compounds that have a broad range of biological activities, such as antibiotic, antiviral, antioxidant, antifouling, anti-inflammatory, cytotoxic and antimitotic activities (Salvador et.al., 2007). Seaweed constitutes commercially important renewable resources. The ability of seaweeds to produce secondary metabolites of potential bioactive compounds of interest have been extensively documented. The only significant class of polyphenolic compounds from marine plants is ‘Phlorotannins’ or algal polyphenolic compounds, which are only know from brown algae (Phaeophyta) (Swanson and Druehl, 2002). Brown algal compounds (Kuda et al., 2007), (Shibata et.al. 2006). Gracilaria sp. Also Decreases in blood glucose concentrations (Murata and Nakazoe et al., 2001)
Marine algal group signifies a gigantic source of nutrients supplements with brilliant structures and also offers huge potent biological activity. Seaweeds has been utilized as a novel nourishment with potential dietary advantages in the food industry and also a solution for different medicinal purposes in pharma field (Santoso et al., 2004). Around 2400 or more marine natural components have been isolated or extracted from different species of seaweeds of subtropical and tropical populations (Manilal et al., 2009). Recent discoveries confirm that seaweeds can act potentially as antiviral, antibacterial, antifungal and antitumor and so on.
2.2 PROBLEMS IN AQUA-FARMING AND ADVANTAGE OF SEAWEEDS OVER IT:
The issues in the aquaculture farming are typically arisen by prevention illness outbreaks or by treating the infection with medications or chemicals. The utilization of antimicrobial chemicals has expanded altogether in aquaculture field (Alderman and Michel 1992). Anti-bacterial agents utilized as a part of both human and veterinary solutions have been attempted experimentally to treat fishes and shrimps from the bacterial infection. Issues with this chemical antibiotic including solvency, toxicity, palatability, cost, delivery and government restriction have constrained the accessible anti-bacterial to a chosen few, particularly in contrast with aquaculture culture. Diminished adequacy and resistance of pathogens to anti-bacterial agents has required advancement of new options (Smith et al. 1994). Numerous pharmacologically active and bioactive dynamic substances have been segregated from seaweeds. For example, marine algae or seaweeds extracts were accounted for to show antibacterial action (Siddhanta et al. 1997, Mahasneh et al. 1995, Sachithananthan and Sivapalan 1975). Many researchers had discovered antibacterial actions of microalgae because of fatty acids (Cooper et al. 1983; Findlay and Patil, 1984; Viso et al. 1987; Kellam et al. 1988). Changyi et al. (1997) opined that the unsaturated fats (PUFA) in litter fall of mangroves may have positive part on the development of fishes and shrimps
Srinivasa Rao and Parekh (1981) demonstrated that some of the crude extracts seaweeds located over geographical region Indian were showing activity just against Gram-positive microorganisms. Ethanol-extracted seaweeds from 56 Southern African seaweeds from the divisions Chlorophyta (green), Phaeophyta (dark colored) and Rhodophyta (red) scored most astounding antibacterial action for Phaeophyta (Vlachos et al. 1997). Comparable outcomes were accounted by Caccamese and Azzolina (1979) and Pesando and Caram (1984) for screening thinks about on kelp of Mediterranean and Eastern Sicily coast respectively. Despite the fact that literature of various investigations on the bioactivity of acquitic flora, our work on testing the antibacterial efficacies of these on fish and shrimps pathogens was all over a new idea and very little endeavor had been made before in this sector. In past reviews, we recorded the adequacy of sponge and coelenterates (Choudhury et al. 2002 and 2003) for their antibacterial study and its activity against pathogenic microbes of fish and shrimps.
2.3 SEAWEEDS AND ITS BIOACTIVITY:
Many reviews, on various ocean seaweeds species, have affirmed their healthful importance around the world. Being specific, seaweed contains low calories, it is a substance with dietary fibers, and they are a decent source of polyunsaturated fatty acid DHA and EPA and may have proteins up to 44% dry matter with an amino acid profile of intrigue (Holdt and Kraan, 2011). The red and the green kelp are in generally rich in carbohydrates, while the brown kelp is richer in soluble fiber and iodine (Gupta and Abu-Ghannam 2011a). Sometimes, some basic amino acids may restrain, as tryptophan, while the grouping of other amino acids, such as taurine, can be highly specific in red algae (Dawczynski et al., 2007). Addition to their nutritious values, seaweeds display fascinating pharmacological properties, for example, anticancer properties, antimicrobial properties, anti-inflammatory and also antioxidant properties (El Gamal, 2010; Gupta and Abu-Ghannam 2011a; Gupta and Abu-Ghannam 2011b; Holdt and Kraan 2011; Mohamed et al., 2012). The active compound is naturally incorporate with phytochemicals (e.g. phlorotannins) minerals, lipids, peptides, and carotenoids it also includes polysaccharides (e.g. fucoidan) (Gupta and Abu-Ghannam 2011b; Holdt and Kraan 2011). It is worth to say that some of these mentioned compounds such as phlorotannins are not found in earthly (terrestrial) plants. Seaweed most abundant source of bioactive material. The antibacterial activity found in seaweed extricates has been noticed and accounted since 1917. biological active compounds removed from some kelp species, to be specific, Phaeophyceae, Rhodophyceae and Chlorophyceae, were demonstrated to have potential restorative activity, for example, antibacterial, anti-mosquito, antiviral, antitumor, hatchling control, antifungal and antiprotozoal(Reuter, H.D et.al 1996). Till the recent date, the just certain anti-bacterial activity of seaweed species has been contemplated, reported and studied in detail [evaluation of minimum inhibitory concentration (MIC) and least bactericidal concentration].Brown ocean seaweeds like
Dictota (Lawson L.D1998) and Sargassum (Pai S.T., Platt, M.V., 1992) have been considered and they demonstrated promising antibacterial action. Compounds having groups phenolic which assume a noteworthy part in antibacterial and antifungal action are discovered richly in brown seaweed when contrasted with the green and red kelp(Sørum, H et.al 2002). Seaweed is considered as a wellspring of bioactive compound with cytostatic, antiviral, antihelminthic, antifungal and antibacterial exercises. They have likewise been utilized to treat a few ailments like a disease, joint inflammation and so on. Seaweed is the inexhaustible living sources which are likewise utilized as sustenance, feed and compost in many parts of the world. They have been screened broadly to isolate life-sparing medications or biologically active substances everywhere throughout the world (Cabello, F.C., 2006).
2.4 ANTIOXIDANT ACTIVITY OF SEAWEEDS:
Marine algae are thought to be a rich energy and also contain antioxidant property, for example, carotenoids, polyphenols, enzymes, pigments and assorted multifunctional polysaccharides. Like all photosynthesizing plants, marine algae is additionally exposed to a combination of light and high oxygen concentration, which prompts to form free radicals and other strong agents. The seaweeds do not show the presence of any damage proposes that their cells have some defensive antioxidative mechanisms and compounds (Matsukawa et al., 1997). Seaweeds additionally create different sorts of antioxidant to counteract environmental stresses (Lesser et al., 2006). Consequently, they can be considered as a potential wellspring of novel material that containing antioxidant agents.
Amin Ismail et al., 2002 assessed the antioxidant activity of some seaweeds accessible in the Malaysian supermarket. Four sorts of seaweeds use to be specific Nori (Porphyra sp.), Kumbu (Laminaria sp.), Wakame (Undaria sp.) and Hijiki (Hijikia sp.) were utilized as a part of his study. The extraction was carried out with water and ethanol, respectively. The β-carotene bleaching and 1,1-diphenyl-2-picrylhydrazyl (DPPH) tests were utilized to see the presence of antioxidant properties of seaweeds by measuring the decline in the graph of absorbance at 470 and 517 nm. In water extract of seaweed, Kumbu demonstrated the most elevated activity antioxidant of up to 63% contrasted to different samples of seaweeds. Kumbu, Nori and Hijiki displayed higher radical scavenging property than Wakame when extraction process is carried out with water. Wakame showed the highest free radical scavenging activity and antioxidant in ethanolic extraction process with 58% and EC50 = 0.42 mg/ml individually. The outcomes his research demonstrated that commersial seaweeds show different variation in antioxidant properties.
2.5 ANTIOXIDANT AND ANTIBIOTIC ACTIVITIES OF SEAWEED:
Sanaa M.M. Shanab 2007 showed the activity of antioxidant by using DPPH strategy (free radicals scavenging) and lipid peroxidation (Fe2+/Ascorbate) , by using three distinct types of seaweeds species namely Laurencia papillosa, Sargassum dentifolium and Jania corniculata). All these seaweeds were gathered from place Defressoar in 2004. Every species of seaweed were extracted using ethanol or Dichloromethane and different range of concentration was made. An antioxidant activity action was found to be higher in dichloromethane extraction of algal species than that of ethanol extraction utilizing both free radical scavenging and lipid peroxidation test. The most extreme free radical scavenger activity was shown by a higher concentration of dichloromethane seaweeds extract of S. dentifolium taken after by L. papillosa and then J. corniculata. Additionally, a higher amount of concentration of dichloromethane seaweed extract of L. papillosa had the most extreme anti-lipid peroxidation action taken after by S. dentifolium and then J. corniculata. All the seaweed extract indicated anti-bacterial and anti-fungal action against four bacterial and two fungal species. Spectrophotometric and chromatographic carried out of these extract assured of the dynamic presence of an antioxidant and anti-bacterial that may be credited to the algal content of chlorophylls, carotenoids and free phenols and also fatty acids
2.6 VIBRIOSIS AND OTHER INFECTIONS IN AQUACULTURE:
“Vibriosis” is a general term used in contrast to disease brought on by various species of Vibrio. A few researcher like Brunn and Heiberg, 1932, 1935; Ljungberg, 1963 detailed vibriosis from different parts of the globe. Vibriosis is widely spread across the globe which, not just causes the tremendous financial decline to the marine fish culture industry but additionally also act significantly in causing disease which affect cultivating of shrimps in Southeast Asia and Japan (Adams etal., 1999).
Diseased and health cultured marine finfishes have a comparable bacterial flora principally having a place with the family Vibrionaceae (Wong and Leong, 1987). The family Vibrionaceae is 10 an indigenous group of microbial biota of marine and estuarine conditions constituting 0.1 to 60% of the aggregate heterotrophic microscopic organisms (Simidu and Sukamoto, 1985). The predominance of Vibrionaceae in healthy seen fishes was high and demonstrated no distinctions characters from those of unhealthy fish. From all the vibriosis outbreaks around the globe, V. anguillarum has been found maximum time in diseased (Schaperclaus, 1927, Nybelin, 1935). Vibrio anguillarum is an outstanding fish pathogen that causes vibriosis infection in brackish and marine water fishes (Anderson and Conroy, 1970; Home, 1982). Eventhough there are a lot of occurrences of vibriosis around the world, the writing relating to vibriosis among fish is thoroughly ailing in Indian inland and seaside waters (Georgekuuy, 1995).
Vibriosis is a typical disease that affects warm and cold water fish and seafood species brought on by microscopic organisms of the genus Vibrio, for example, Vibrio anguillarum, V. ordalii, V. harveyi, V. vulnificus, V. parahaemolyticus, V. alginolyticus, V. salmonicida, which can aggregate in the fishes and shrimps body. Treatment with available anti-bacterial drug can cause poisonous effects over the shrimps and fishes and the release chemical residues deposits into the nature. Some Vibrio strains, namely V. harveyi, V. splendidus and V. parahaemolyticus are resistant to a various type of anti-bacterial drugs. Cavallo et al. (2013) watched distinctive susceptibilities of the chloroform and methanol extraction of red and green seaweeds on various Vibrio species by carring out antimicrobial assay using dsc diffusion technique. G. longissima offered the broadest range of antibacterial spectrum, demonstrating action against many vibrio species namely V. alginolyticus V. ordalii and V. vulnificus, trailed by Cladophora rupestris extract, showed dynamic activity against three species, and Chaetomorpha linum and G. dura extricates against two species, yet none of the extract tried exhibited restraint against V. splendidus and V. harveyi.
Thanigaivel et al. (2014) affirmed that the ethanol extraction of Chaetomorpha antennina in vitro by the agar well diffusion technique and in vivo was carried on shrimp’s infection with Vibrio parahaemolyticus. The ethanol extraction of C. antennina was exceptionally successful in controlling the infection caused by V. parahaemolyticus, which was found to be resistant to ampicillin and delicate to erythromycin.
Thanigaivel et al. (2015) affirmed in vitro study of antibacterial activity of ethanol extract and aqueous extract of different seaweeds Padina gymnospora, Gracilaria folifera, Sargassum cinereum and S. longifolium by means of the agar well diffusion technique and in vivo in fingerlings of Oreochromis mossambicus (tilapia) by making them infected with Pseudomonas aeruginosa and dectection study was carried out by utilizing PCR.
Kavita et al. (2014) studied the methanol extraction of 38 seaweeds and was tests against human pathogenic microorganisms, and Gram-positive namely S. aureus and B. subtilis and Gram-negative namely E. coli and P. aeruginosa. Laurencia papillosa indicated greatest antibacterial action, and the active dynamic fraction was recognized as a cholesterol subordinate, 24-propylidene cholest-5-en-3 beta-ol. The MIC values against clinical sample isolated ranged from 1.2 to 1.7 µg/mL.
Thanigaivel et al. (2015) performed a study to show the antibacterial protection for fish and shrimps by immersion technique. Accordingly, initiated disease in fish and shrimps with serially diluting (101 to 105) of pathogenic microbial cell suspension were poured into the water tank where healthy fish and shrimps were kept. For treatment diverse concentration changing from values, 50 mg/L to 500 mg/L of prepared seaweeds extract used in his study was included in the experimental tanks. Symptomatic or dead animals were recorded.
Krish and Das (2014) study revealed that the methanol, ethanol and ethyl acetic acid crude extract of Cladophora rupestris gathered in a Mediterranean territory, demonstrated great antimicrobial action against various vibrio species namely V. harveyii, V. parahaemolyticus and V. alginolyticus, which were studied by the agar diffusion technique and the zone of clearance was observed. The fatty acid profile demonstrated oleic, alpha linolenic, palmitic, myristic, palmitoleic and linoleic acids. Albeit scarcer, study of other algal parts have been accounted. Tanniou et al. (2014) tried the antibacterial action of phenolic items from Sargassum seaweeds against Vibrio aestuarianus, V. anguillarum and V. parahaemolyticus and found positive results
2.7 SEAWEED FOR SAFETY AND QUALITY ATTRIBUTES OF FOODS:
The production of rancid flavors and odors is because of oxidative stress created in the food can prompt a decrease in the sensory attributes, dietary nutritional quality and food safety. Because of buyer requests, intrigue has been created in hunting plant items down for normal “green” additives. Macroalgae or seaweed extract are rich in the polyphenolic compound which have been reported many times for their antioxidant properties. They additionally have antimicrobial actions against most significant food spoilage and food pathogen. Along these lines, the likelihood of seaweed being added to nourishments as a wellspring of antioxidant agent and antimicrobial is the primary concentration of this correspondence. What’s more is the seaweeds are additionally rich in dietary minerals particularly sodium, potassium and iodine. Another potential zone where the utilization of seaweeds is picking up its significance is in regards to their expansion for enhancing the textural properties of food items which are likewise broadly checked on in this paper (Shilpi Gupta et al., 2011).
Modern importance: The pattern towards the utilization of “normal green” plant and other forms like seaweeds in different foods and drinks in the nourishment business is picking advantage. Seaweeds, being a rich wellspring of fundamentally various bioactive mixes with important nutraceutical properties, can be utilized as an ingredient to supplement food with the functional compounds. Enthusiasm for the utilization of such mixes as a cancer antioxidant agents, antimicrobials or texture agents in various nourishment items is more noteworthy than at any other time. The expansion of kelp or their extract to food and drinks items will lessen the usage of chemical additives for preservation process, which will satisfy the business and in addition buyer requests for “green” product. Furthermore, the flow status and the future projections in the practical impacts of ocean growth as a way to enhance the fiber and decrease the salt substance of food items, which will be of noteworthy significance to the meat business. (Shilpi Gupta et al., 2011).
3. MATERIALS AND METHODS
3.1 MEDIA AND CHEMICALS:
Mueller Hinton agar, Nutrient broth, Nutrient agar, 1N Hydro chloric acid, Xanthum gum, Sodium caseinate, Glycerol, Formalin, Ethanol, Dichloro-methane, Chloroform, petroleum ether, Dimethyl sulfoxide, Sodium carbonate, Acetonitrile, Acetone, Sodium Chloride, Disodium Phosphate, Potassium Potassium Chloride, Sephadex G50, Trichloroacetic Acid, Potassium acetate, Aluminium chloride, Sodium phosphate, Sulphuric acid, Hexane, Potassium Ferricyanide, Ferrozine, Hydrogen Peroxide, Ferrous Sulphate, Sodium Salicylate, DPPH, ABTS, APS, Ammonium molybdate, Ferric chloride,. Phosphate buffer saline (PBS), Ascorbic acid, Folin-ciocalteu reagent, Gallic acid, Quercetin, EDTA, Every chemicals utilized were of standard grades and acquired from Sigma chemicals.
3.2 GLASS WARES AND PLASTIC WARES:
Test tubes, 15 ml centrifuge tubes, 100 ml conical flask, 250 ml conical flask, 500 ml conical flask, 50 ml centrifuge tubes, micro centrifuge tubes (1.5 ml and 2 ml), microfuge tubes 5 ml and glass beakers, 250 ml side arm flask, autoclavable cap bottols (500 ml and 2000 ml) all the glass wares and plastic wares were purchased from Tarsons and Borosil Glass Works Ltd.
3.3 COLLECTION AND MAINTAINANCE OF SEAWEED:
The algal species Sargassum tenerrimum were gathered from the seaside territory of Rameshwaram, India. The seaweeds under study was handpicked and washed completely with seawater to expel undesirable impurities stick to it like sand particles and epiphytes. They were transported to the research center in polythene packs. The collected sample of seaweed was then washed altogether utilizing tap water to evacuate salt on the surface of the test seaweed (Sivasankari et al., 2006). The cleaned seaweed sample was then dried in hot air oven at 50-60 °C for 72 hr. The dried sample of test seaweed was then kept in air lock plastic pack at a dry and cool place to keep them free from remoisturizing (Nurul Aili Zakaria et al., 2011). The dried specimens were cut into little pieces and crushed into fine powder utilizing a grinder. The tests sample of seaweed were sieved to get uniform small particles, then kept in a sealed air tight container and sealing the cap with parafin tape to avoid entry of air and put away to store in a cooler condition (–20⁰C) until further examination (Amin Ismail et al., 2002).
3.4 COLLECTION AND MAINTENANCE EXPERIMENTAL ANIMAL:
Freshwater prawn (Macrobrachium rosenbergii) were in healthy condition and were collected from aqua ranch situated in Chengalpet. They were transported to the laboratory in live condition in 20L tanks with air circulation and fresh water. In the research aquaculture laboratory, they were transferred from 20L tank to in 1000L fiberglass tanks with fresh water and loaded with complete aeration setup and water inlet and outlet system. The animals were given healthy fed twice a day and water in tanks were cleaned once in every week.
3.5 BACTERIAL CULTURE
Bacterial culture used as a part of the project was Vibrio parahaemolyticus and was isolated from the infected shrimp. The bacterial culture was cultured on Nutrient agar plate with 2% NaCl. This was utilized for the pathogenicity tests. The pathogenicity of the bacterial isolate was studied by immersion (bath exposure) and intramuscular methods. The technique of (Ducklow et al., 1980) was taken in practice.
3.6 MOISTURE CONTAIN OF SEAWEED
This method is used to determine the percentage of moisture in a sample by drying the sample to a constant weight. 1 gram of test seaweed powder was taken in a glass vial. Then the total weight was measured. The glass vial containing seaweed powder was kept in hot air oven at 92⁰C for 3 hours. Allowed the sample to cool, then we measured the weight was the cooled sample.
The moisture content was determined using following formula:
 %W = x –y × 100
%W = x –y × 100
X
Where:
%W = percentage of moisture in the sample
x = initial bottle weight with sample
y = final bottle weight with sample
X = sample weight
3.7 PREPARATION OF SEAWEED EXTRACTS
Around 5 grams of finely crushed seaweed as described above were used for the extraction process. The technique used was maceration. 100 ml of each solvent were taken namely chloroform, Dichloro-methane, Ethanol. Petroleum ether and water (Hayman) was used for the study. Each of this solvent was added with 5 grams of the finely crushed seaweeds. It was kept for shaking incubation of 48 hours under dim light condition. After incubation, the extract in the each of the solvent was filtered using whatman filter paper number 1 and the filtrate was then evaporated using rotary evaporator set up to obtain crude extract powder. (De et al., 1997). The seaweed crude extract obtained from evaporations was then split into two half of each of the solvent extract and all the parts were re-suspended with respective solvent. One half part of each solvent extract was kept as crude and other half of each extract was kept for purification.
3.8 PHYTOCHEMICAL ANAYLSIS
Phytochemical analysis of seaweed extract was done by the following standard procedures
by (Imran et al. 2010; Trease and Evans, 2002; Sofowora, 1993; Harborne, 1973; Olabinri et al 2014; Sadasivam and Manickam, 1996; Premalatha et al. 2011). The prepared seaweed extracts were subjected to perform following preliminary phytochemical screening test.
3.8.1 Test for tannin:
Tannin in the seaweed extract was determined by adding few drops of 0.1% FeCl3 to 1 ml extract. Presence of bluish black indicates tannin.
3.8.2 Test for flavonoid:
Flavonoid identification was carried out by adding, 2 ml of seaweeds extract, 1 ml of 2N sodium hydroxide was added. Presence of yellow color indicates the presence of flavonoids.
3.8.3. Test for phenols:
For phenol identification, 1 ml of the seaweed extract, 2 ml of distilled water followed by few drops of 10% ferric chloride was added. Formation of blue or green color indicates presence of phenols.
3.8.4 Test for Coumarins;
For coumarins identification, 1 ml of seaweed extract, 1 ml of 10% NaOH was added. Formation of yellow color indicates presence of coumarins.
3.8.5 Test for Phlobatannin;
Phlobatannin detection, 1 ml of 1% Hydrochloric acid was added to 1 ml seaweed extract and the mixture was kept in water bath for 10 min. Formation of a red color precipitate indicates the presence of phlobatanin.
3.8.6 Anthraquinones:
Anthraquinone identification was carried out by adding, 1 ml of seaweed extract to which few drops of 10% ammonia solution was added, appearance of pink color precipitate indicates the presence of anthraquinones.
3.8.7 Test for saponin:
Saponin identification, frothing test was employed 2 ml seaweed extract, 2 ml of distilled water was added and the mixture was kept in the shaker for hour. Formation of foam confirms the presence of saponin.
3.8.8 Test for alkaloid:
Alkaloid identification was performed by the modified quantitative method of (Subathraa and poonguzhali, 2013) 1 ml of seaweed extract, 1 ml of concentrated hydrochloric acid was added. Then few drops of Mayer’s reagent were added. Presence of green color or white precipitate indicates the presence of alkaloids,
3.8.9 Test for carbohydrates:
Mix 2 ml of seaweed extract, 1 ml of Molisch’s reagent and few drops of conc. sulphuric acid were added. Purple or reddish color indicates the presence of carbohydrates.
3.8.10 Test for cardiac glycoside:
Glycoside identification, legal test was used. To 1 ml of seaweed extract, 1 ml of glacial acetic was added, followed by few drops of FeCl3 solution, and 1 ml of concentrated H2SO4 addedslowly, near the side of the test tube a brown ring was formed is positive for cardiac glycoside.
3.8.11 Test for quinines:
For quinine identification, 1 ml of seaweed extract, 1 ml of concentrated sulphuric acid was added. Formation of red color indicates presence of quinones.
3.8.12 Test for triterpenoids:
Triterpenoid identification by carried out the addition of 1.5 ml of seaweed extract, 1 ml of Libemann-Buchard reagent was added. Formation of blue green color indicates presence of triterpenoids.
3.8.13 Test for glycosides:
For glycoside identification, 2 ml of seaweed extract, 3 ml of chloroform and 10% ammonia solution was added. Formation of pink color indicates presence of glycosides.
3.8.14 Test for proteins and aminoacids;
Identification for ninhydrin test was followed 2 ml of seaweed extract, few drops of 0.2% Ninhydrin was added and heated for 5 min. Formation of blue color indicate the presence of proteins.
3.8.15 Steroids and Phytosteroids:
Steroid and phytosteroid identification, 1 ml of seaweed extract equal volume of chloroform is added and subjected with few drops of concentrated sulphuric acid appearance of brown ring indicates the presence of steroids and appearance of bluish brown ring indicates the presence of phytosteroids.
3.9. ANTIOXIDANT ACTIVITY:
Seaweeds which was dissolved in different solvents and extracted were used to carry out antioxidant assay.
3.9.1 Determination Total Phenolic Content
The aggregate of polyphenolic concentration was measured by Folin-Ciocalteau strategy. (Schlesier, Harwat, Bohm, and Bitsch, 2002, Chandler and Dodds, 1983). In this technique, 10 µl each of seaweed extract that is extracted in chloroform, dichloromethane, ethanol, petroleum ether (all processed by Maceration technique) were taken into 1.5 ml microfuge tubes and each was added with 20µl of Folin-Ciocalteu reagent and they were allowed it to remain still for 2 minutes. After this 50 µl of sodium carbonate and 920 µl of distilled water was added to the mixed well and incubated at the dark condition for 60 minutes. The specimen absorbance was noted utilizing an UV Bio photometer at absorbance range of 725nm. Standard utilized for total phenolic content was gallic acid. (mg of Gallic corrosive/g) (Walailuck Boonchum et al., 2011).
3.9.2 Determination of Total Flavonoid Content
This technique was utilized to determine of an aggregate of flavonoid content in the seaweeds extracts use in this study, which was from Yafang et al., (2011). 10µl of the all the seaweeds extract were included with 365µl of 95% ethanol, in this 25µl of Aluminum chloride, 25µl of potassium acetic acid derivation and 700µl refined water was added up in 1.5 ml microfuge tubes. The described mixture was kept in the dark condition chamber for around 30 minutes. Absorbance for this assay was checked at 415 nm against a blank sample. Standard used for this assay was Quercetin (Cox et al., 2010).
3.9.3 Determination of Total Antioxidant Activity
Calculation of total antioxidant activity of seaweeds exetacts were carried out using Phospho molybdenum as indicated by the technique of Prieto et al., (1999). The antioxidant can lessen from Mo (IV) to Mo (V) and the green phosphate/Mo (V) compound which is calculated at absorbance 695 nm, were made (thoudam bhaigyabati et al., 2011).
10 µl of tests sample of seaweeds was mixed together with 90µl of ethanol, in which 1ml of reagent solution prepared in concentration proportion of 1:1:1 sulphuric acid (1.66g/50ml water), ammonium molybdate (0.247g/50ml water) and sodium phosphate (0.532g/50ml water) was added. This above mixture was kept for incubation in water-bath tank at 98⁰C for one hour and thirty minutes. After the incubation of the mixture it was allow to cool at room temperature for 10 minutes. The absorbance was reading for mixture was carried out at 695 nm by utilizing an UV Bio photometer. Ascorbic acid was utilized as a standard. Total antioxidant activity is expressed as the number of equivalents of ascorbic acid in milligrams per grams of the extracts.
3.9.4 Determination of free radicals scavenging by DPPH method
 Determination of free radicals scavenging by DPPH method was carried out using the modified conventional protocol by Brand-Williams, Cuvelier, and Berset (1995). Free radical scavenging activity of the seaweeds extract was tested and recorded by using,1-diphenyl-2-picryl hydrazyl (DPPH). DPPH mixture was made by adding 7.8 mg in 100 ml of ethanol. 5 µl seaweed extract was mixed together with 495 µl of ethanol and 500 µl of 0.1 mM DPPH (Thoudam bhaigyabati et al., 2011). The above test mixture was incubated at room temperature in dimmed light condition for around for half an hour. The absorbance reading of the test mixture was carried at 515 nm utilizing an UV-spectrophotometer. For blank, seaweeds extract was not included in tubes and rest all reagents were added and Quercetin was utilized as a standard. The percentage DPPH was computed utilizing the formula given below:
Determination of free radicals scavenging by DPPH method was carried out using the modified conventional protocol by Brand-Williams, Cuvelier, and Berset (1995). Free radical scavenging activity of the seaweeds extract was tested and recorded by using,1-diphenyl-2-picryl hydrazyl (DPPH). DPPH mixture was made by adding 7.8 mg in 100 ml of ethanol. 5 µl seaweed extract was mixed together with 495 µl of ethanol and 500 µl of 0.1 mM DPPH (Thoudam bhaigyabati et al., 2011). The above test mixture was incubated at room temperature in dimmed light condition for around for half an hour. The absorbance reading of the test mixture was carried at 515 nm utilizing an UV-spectrophotometer. For blank, seaweeds extract was not included in tubes and rest all reagents were added and Quercetin was utilized as a standard. The percentage DPPH was computed utilizing the formula given below:
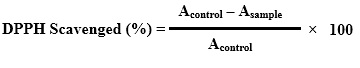
Where,
Acontrol = Absorbance of DPPH radicals without samples,
Asample = Absorbance of DPPH with samples.
3.9.5 Hydrogen Peroxide Scavenging Capacity
The capability of the seaweeds to scavenge H₂O₂ was studied by taking after the technique by Ruch et al., 1989 with the slight change of Gulcin et al., 2003. 10 ml of H₂O₂ solution was made by adding 9950 µl of phosphate buffer and 49.3 µl of H₂O₂. 10µl of the seaweed extract was added together with 2799 µl of phosphate buffer and 600 µl of H₂O₂. The absorbance of H₂O₂ scavenging study was recorded utilizing UV-visible spectrophotometer at 240 nm against a blank which contains only phosphate buffer without H₂O₂. Quercetin was utilized as a standard for H₂O₂ study. The scavenging impact of H₂O₂ was figured by utilizing following formula:
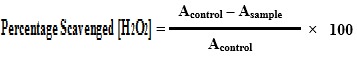
Where,
Acontrol = Absorbance of H₂O₂ radical without adding sample,
Asample = Absorbance of H₂O₂ radical with samples.
3.9.6 Hydroxyl radical scavenging assay
Hydroxyl radical scavenging assay of seaweeds extract was carried out by utilizing the technique of Costa et al., (2009). In this procedure, 10 µl of the seaweeds extract was included with 150 μl sodium salicylate, 350 μl H2O2, and 500 μl FeSO4. On account of control, the each seaweeds extract was exchanged with respective individual solvents. In A2, sodium salicylate was changed to H2O. All the reaction mixture added with reagents was incubated at 37°C for 60 minutes. Absorbance of the test mixture was measured at 562nm utilizing an UV Biophotometer. Distilled water was utilized as a blank for assay. Quercetin was utilized as the standard for this assay. The scavenging impact was computed in percentages by utilizing the specified formula given below:
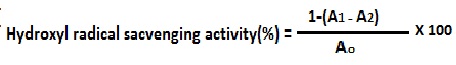
A1 Regents and extract, Ao control , A2 reagents and distilled water
3.9.7 ABTS radical scavenging assay
The ABTS (2, 2′- azino-bis3-ethylbenzthiazoline-6-sulfonic) radical scavenging assay was carried out for all seaweeds extracts by using method of Re et al. (1999) with some particular alterations. The ABTS reagent for the assay was made by adding up 10 mg of ABTS in 5 ml of distilled water. 2.2 mg of APS (Ammonium Per sulfate) was added up in 5 ml of distilled water. Above prepared mixture of ABTS and APS were put together and mixed well and made the total volume up to 50 ml by adding up with distilled water. Then the prepared ABTS mixture was kept for six hours incubation in the dark conditions. After the incubation of six hours, 10 µl from test seaweeds sample was added up with 1 ml of ABTS solution and 90 µl of ethanol. The absorbance test mixture was measured at 734 nm utilizing UV-spectrophotometer. Trolox was utilized as a standard for this assay. The calculation of ABTS radical scavenging was carried out using following formula:
 % Inhibition = A × 100
% Inhibition = A × 100
A₀
A express the absorbance of sample at 7 minutes kinetics; A₀ express reference (Walailuck Boonchum et al., 2011).
3.10 CHARACTERIZATION OF ACTIVE COMPOUNDS
3.10.1 GC–MS analysis for bioactive compounds:
GC–MS is gas chromatography – mass spectrometry. This is a used for identification of bioactive compound present in seaweed extract. The Hamilton syringe was used to inject sample of volume 2.0 µl in to the GC-MS manually for total ionchromato-125 graphic analysis in split mode. In quantitative analysis, selected ion monitoring mode was employed during the GC–MS analysis (Thanigaivel et al., 2014; Thomas et al., 2014)[2][6].
3.10.2 HPLC analysis:
HPLC is an easily adaptable, powerful, and broadly utilized technique for the segregation of common natural compounds (Cannell, 1998). At present, this system characterization is picking up its value and demand among different expository technique as the primary decision for fingerprinting study for the quality control of herbal and medicinal and other materials like seaweeds etc. (Fan et al., 2006). Purification of the compound of interest utilizing HPLC is the way toward isolating or separating the objective compound from other (structurally related) mixes or contaminants. Each compound in this characterization process should contain a unique peak under specific chromatographic conditions. Depending upon what we have to isolated and how firmly related the samples are, the chromatographer may pick the conditions that are required to obtain those specific conditions, for example, the mobile phase, run time, flow rate, comprehensive detectors and column to get the best separation. Identification of compounds is a crucial part when HPLC assay is taken in consideration. To identify certain compounds by HPLC, we have to select the best detector for the study to obtain best results. Once the detector is chosen for HPLC assay and is set to ideal detection settings. The parameters of this assay ought to be with the end goal that a perfect peak of the known sample is seen from the chromatograph. The distinguishing peak obtain in process should have a reasonable retention time and ought to be very much isolated from extraneous peaks at the recognition levels which the assay will be performed. The samples initially treated in such a way that the components of interest are efficiently liberated into the solution.
The samples were injected in the column by using a standard syringe used for HPLC. The mobile phase, run time, the flow rate was set as per the requirement of each sample. From the result obtained during process we can easily identify the major components that are present in the seaweed extract samples.
3.10.3 FT-IR analysis:
Fourier transform infrared spectroscopy (FTIR) is used to obtain an infrared spectrum of emission or absorption of a gas, liquid or solid. The, IR spectra of the chromatography and crude extract was measured by employing FT-IR spectrophotometer at room temperature using KBr, and was measured in the spectral range of 500-4000cm-1for functional group analysis.( Sangappa et al.,2013).
3.10.4 FT-RAMAN analysis:
Fourier-transform Raman spectroscopy was carried out by utilizing a “Bruker IFS 66 instrument with a FRA 106 Raman module connection and a Nd3+ /YAG laser effective at 1064 nm” in the near infrared. The liquid samples of seaweeds extracts showing good results was carried out for this characterization (Crude and purified for both Dichloromethane and Ethanol) were studied in aluminium cups with a spectral “footprint” of 100 m and the spectra were noted at a 4cm−1 the spectral resolution over the wavenumber range 50–3500 cm−1. To improve the spectral signal-to-noise ratio, 2000 scans were gathered over a period of about 30 min in replicate. All spectra were got more than twice from each position and no distortion or fluctuation in band positions and intensities were recorded. Comparison of the bands in the specimens was done by the spectra noted before for a project. The obtained results in this are compared with the control samples.
3.11 IN-VITRO STUDY OF ANTIBACTERIAL ACTIVITY OF SEAWEED EXTRACTS
3.11.1 Preparation of experimental media:
The crude seaweed extract (1 mg) acquired from test seaweed material was first mixed and dissolve up in 0.2 ml of ethanol, dichloromethane, chloroform, petroleum ether respectively at the particular concentration of 1000 ppm. Polysorbate 80 (Tween 80, Qualigens) was utilized as an emulsifier at the 0.05% concentration of the last test solution. Ethanol and polysorbate 80, chloroform and polysorbate 80, dichloromethane and polysorbate 80, petroleum ether and polysorbate 80 all combination were set up as control (Saxena and Yadav, 1983)[4].
3.11.2 Well diffusion method:
Muller Hinton Agar medium was made and sterilized in autoclaved. The test creature was transferred into a test tube containing 5 milliliters of sterilized nutrient broth from the stock culture with help of nichrome wire circle. The broth inoculated with culture was incubated to get moderate turbidity for about 10-13 hours at 30-degree centigrade. The agar well diffusion technique (Perez et al., 1990) [5] was taken after. A sterile cotton swab was plunged into the stock culture inoculum. The cotton swab was then turned squeezing against the wall of the tube, to remove excess inoculum. The surface of Muller-Hinton agar plate was swabbing three times by turning the plate by 60-degree angle between swabbings. Four wells of 8 mm width were bored in the agar on each plate use for different solvents respectively utilizing a clean well cutter. Into each well, (20, 30, 40, 50 µl) of the seaweed crude extract was added. The extract in the well was allowed to diffuse for 2 hrs. The plates were incubated at 30º C for 24-48 hours. The antibacterial activity was observed and recorded and the zone of inhibition was measured around the wells.
3.11.3 Preparation of seaweed extract disc:
All the solvent extract namely chloroform, dichloromethane, ethanol, petroleum ether was use to prepare disc. (30 µl) of the ready seaweed extract was added to the sterilized disc (Hi media) and dried under a sterile condition at room temperature. The disc was utilized as a part of the study.
3.11.4 Disc diffusion method:
The technique of Bauer Kirby (1966) was undertaken. Muller Hinton agar plates were prepared utilizing aseptic procedures and lawn of bacterial culture was spread over using the swabbing technique. The prepared disc of different solvent seaweed extract was placed in the center of the plate. Each disc was pushed down onto the surface of the agar plate by using sterile forceps. The plates were then incubated at 30ºC for 24 hours to record for any of the round clear zone (Zone of Inhibition) around the disc. The width of the zone of clearance was measured. The test was done in triplicates.
3.11.5 Minimum inhibitory concentration (MIC):
The MIC of the seaweed extract carried against Vibrio parahaemolyticus was done by the tube dilution method. 4ml Nutrient broth was suspended in each test tube and autoclaved. The nutrient broth was allowed to cool and differing amount of concentration (10, 20, 30, 40, 50, 60, 70, 80, 90, 100 µg/ml) of the seaweed extract of crude and purified by column chromatography were added to the broth. 100µl of bacterial cultural was inoculated in each of the tube under the study and then tubes were incubated at 37ºC for 14 to 24 hours. After incubation tubes were examined for any of bacterial growth using spectroscopic method. MIC of the extract was recorded by finding out the first test tube inhibiting the growth of given bacterial culture.
3.11.6 Growth kinetic assay:
Growth kinetic assay was carried out to study effect of crude and purified seaweed extract against the pathogen. For this assay, 250 ml of nutrient broth was prepared and dispense 25 ml of it in each ten side arm flask. The prepared flask was kept for sterilization in autoclave. After sterilization each (except blank) flask was inoculated with 100 microliter of bacterial culture which was under study. Leaving aside control and blank other eight flask was added with 40 µl crude and purified of each extract use in study. The reading was taken against the blank for each of the flask using cholorimeter at 620 nanometer in every 2 hours of gap. The obtained result was recorded and graph was plot of absorbance against time.
3.12 EXPERIMENTAL PATHOGENICITY OF BACTERIAL CULTURE
The pathogenicity of Vibrio parahaemolyticus in Macrobrachium rosenbergii was studied in this work by two methods first using intramuscular injection and second using immersion technique according to standard method. (Thanigaivel et al. 2014 and Thomas et al., 2013a).
3.12.1 Preparation of bacterial inoculum:
The isolated colonies developed on Nutrient agar plates with 2.0 % sodium chloride after incubations at 37°C for 24 hours, the isolated colonies were then transferred to freshly prepared 10 milliliters sterile nutrient broth with 2.0% sodium chloride broth was then incubated for 48 hours at 30°C in a shaker. After incubation broth was then centrifuged at 6000 RPM for 10 minutes at 4°C and pellet was resuspended with 1 milliliter of sterile PBS, and the suspension was pipetted up and down to break aggregates and clumps. The acquired suspension was then serially diluted in duplicates for immersion and intramuscular from
10-1to
10-5. From each of this dilution 20µl was use for intramuscular study and 1 ml was used for immersion study.
3.12.2 Infection by intramuscular injection:
The healthy shrimps were screened and three shrimps for every tank were kept in 100-liter limit glass tanks. The water was added with air circulation and it was changed every day. The shrimps were given with synthetic food. Five concentration of bacterial suspension made in duplicate that are
10-1to
10-5 were utilized for this analysis. For shrimp 20 µl of bacterial suspension from each of these five dilutions was infused intramuscularly utilizing 1-ml insulin syringes for intramuscular pathogenicity.
3.12.3 Infection by immersion method:
For immersion 1-liter of water was added in each tank and three healthy shrimp were added to each of this tank 1 ml bacterial suspension of different concentration that are
10-1to
10-5were poured in respective tanks. Control shrimps got just clean saline. The analysis was led in triplicates in each bacterial concentration. Shrimps were checked twice a day for any clinical indications of illness and mortality. Dead shrimps were removed and used for further clinical studies.
3.12.4 Toxic bioassay for post larve:
About 3 g of A. salina cysts was aerated in 15 L capacity white bucket containing 12 L filtered estuarine water (salinity 28%). The air stone placed in the bottom of the bucket to ensure complete hydration of the cysts. After 24 h incubation at room temperature (28-29 °C), hatched free swimming pink colored nauplii were tested for the toxicity bioassay as described by (Ayesha et al., 2010). The collected 40 numbers of Artemia nauplii were transferred into Petri dishes containing different concentrations (20-100 μg/mL) of purified extract. The mortality percentage was recorded after 24 h of the interval for one week. The control group treated without the addition of sample and triplicate experiments were carried out, and the results were expressed as mean ± SD values. At the end of experimental period, the numbers of mobile (viable) and dead nauplii in each Petri dish was counted with a hand lens. Nauplii were considered as dead if they were lying immobile at the bottom of the Petri dishes.
3.13 TREATMENT
3.12.1 Treatment using extract using intramuscular injection and immersion method:
M. rosenbergii was infected with Vibrio parahaemolyticus utilizing the intramuscular strategy as described before. The infected shrimps showing with clinical signs were collected and raised independently in clean new tank of treated water with the concentrate of 250 mg/l of each seaweed extract. The shrimps were collected at different time intervals of 12, 24 and 48 hours of exposure and raised further for twenty-five days in aquarium tanks containing freshwater. The prawns survived at the end of 20 and 25 days were counted to decide the ideal presentable exposure time required for seaweed to recover shrimp from a disease brought on by the microorganisms. On account of intramuscular infusion, 25 μl of concentrate was infused intramuscularly into lower third segment scale of shrimp body to treat the pathogenic infection caused to shrimp. The shrimp were observed frequently during this study.
3.13.2 Encapsulation of crude compound for feed treatment feed:
Sodium caseinate and Xanthum gum were taken in the proportion of 7:1 in to 100 ml of milli-Q water the mixture was made in duplicates. To the each mixture, 1% of glycerol was added. The both the mixture was kept on the hot plate magnetic stirrer. 2 ml of the ethanol seaweed extract was added to one mixture and the second mixture added was added with 1ml petroleum ether extract and 1ml of dichloromethane seaweed extract and was kept for overnight shaking on the hot plate magnetic stirrer. After one-day mixing process the mixture was kept for centrifugation at a 1000 RPM for 2 min, to break the air bubbles. 1N Hydrochloric acid was freshly made and to this, the above mixture was added drop by drop utilizing a 5ml syringe. The round beads got were washed thrice with distilled water and dried. The beads were stored for further study of treatment as feed and SEM analysis.(Vijayabaskar and Shiyamala, 2012).
3.13.3 Treatment using the encapsulated seaweeds extract beads:
Both the bead made by carrying out single (Ethanol) extract and combination (petroleum ether and Dichloromethane) was used in treatment. For this study, Shrimps was infected intramuscularly with 25 μl of
10-3dilution of pathogenic cells. Shrimps tank was then added with 0.1 grams encapsulated beads of seaweeds. During this process animals were observed for 25 days and shrimp food and encapsulated beads of 0.1 grams was added to tanks once in a day. After completion of 25 day result was recorded.
3.13.4 SEM analysis:
Scanning Electron Microscopy (SEM), also known as SEM analysis or SEM microscopy, is used very effectively in microanalysis of solid inorganic materials. Scanning electron microscopy is performed at high magnifications, generates high-resolution images and precisely measures very small features and objects.
3.14 HISTOPATHOLOGICAL INVESTIGATION:
For cutting areas of various sections tissues (gills, digestive tract, hepatopancreas, and muscle) in paraffin, clearing of the tissues and dehydration were done at room temperature. After cutting section tissues were first cleaned or washed in two turns of 70% alcohol for one hour each, dried out for two hours in two turns of 70% alcohol for one hour each, additionally dried out for an hour each in two turns of 80% alcohol, evaluated twice in absolute alcohol and 95% alcohol, cleared through a blend of chloroform and alcohol (1:1 v/v) and afterward passed twice in chloroform for one hour each. Chloroform was favored over xylene as it did not make the tissue turn out to be brittle and become hard. The tissues, after cleaning, were left in a blend of chloroform and paraffin wax (roughly 1:1 v/v) for over overnight at room temperature. Before implanting on the slide, the tissues were impregnated in three changes of paraffin wax with ceresin of 58 to 60oC melting point for one hour each. The transverse section was sliced at 5 to 7 µm thickness utilizing a manual rotatory microtome. After deparaffinised in xylene, the areas were hydrated through alcohol up to 70% and used Harris alum hematoxylin to stain them and counterstained with 1% alcoholic eosin (Preece, 1972). Applying the normal method, recolored segments were dried out through the graded alcohol and mounted with a glass slide and add over cover slip in DPX through xylene.
The histology areas were studied utilizing Carl Zeiss binocular compound magnifying lens microscope. Measurement of cellular sections was taken with Carl Zeiss microscope fitted with an ocular micrometer calibrate scale having its accuracy up to 10 µm. Photomicrographs were brought with the advanced camera (Nikon) appended to Carl Zeiss magnifying lens with projection eyepiece 10 X and objective 10X, 20X, 40X and 100 X. The magnification of the much enlarged prints was figured with stage and ocular micrometer.
3.15 GENOTOXICITY BY COMET ANALYSIS:
This analysis was carried out see the damage caused to DNA in eukaryotic cell. For this assay the slides was scraped with sand paper. The scraped slides were dipped in methanol and dry it for 10minutes and wash it with distilled water and dry. 3 different layers are prepared on the slide –
1st layer- 1% HMA was prepared using 1 gram of high medium agrose in 100 ml of 1X PBS out of which 100 microlitre of 1% HMA is taken on a slide and a coverslip is placed on it. Allow it to solidify by keeping the slides on ice packs. Once agar solidify remove the coverslip and add second layer.
2nd layer- 0.5% LMA – 0.5gm of low medium agar in 100ml of 1X PBS was prepared. 25microlitre of sample was mixed with 75 microlitre of 0.5% LMA and mixture was added upon the first layer and place a coverslip on it. Allow it to solidify. Keep the slides on ice for 10 minutes and remove the cover slip.
3rd layer- 1% LMA – 1gm of Low medium agar in 100ml of 1X PBS was prepared 100 microlitre of 1% LMA is added on the 2nd layer. Allow it to solidify, remove the coverslip.
Immerse the slides in lysis buffer (in a tray) for 2 to 4 hours in dark condition. Then soak all the slides in electrophoresis buffer for 20minutes, then run the electrophoresis at 20 volts for 20minutes.Soak the slides in neutralization buffer for 20 to 30 minutes. After neutralization, add 50microlitre of ethidium bromide and place a coverslip. Observe under fluorescence microscope and atleast a minimum of 100 cells should be counted.
4. RESULTS AND DISSUCATION
4.1 MOISTURE CONTAIN OF SEAWEED
Moisture content in the Sargassum tenerrimum was calculated. Moisture content present in the test sample of seaweed was found to be 20%.(Table 2)
| Calculation | Sargassum tenerrimum |
| Percent sample free from moisture | 80% |
| Moisture content present in sample | 20% |
Table 4: Results of moisture content
4.2 PHYTOCHEMICAL ANALYSIS OF SEAWEED
| SR.NO | PHYTOCHEMICAL TESTED | RESULTS |
| 1 | Tannins | Positive |
| 2 | Flavonoid | Positive |
| 3 | Phenols | Positive |
| 4 | Coumarins | Negative |
| 5 | Phlobatannin | Negative |
| 6 | Anthraquinones | Positive |
| 7 | Saponin | Negative |
| 8 | Alkaloids | Positive |
| 9 | Carbohydrate | Negative |
| 10 | Cardiac glycosides | Negative |
| 11 | Quinines | Positive |
| 12 | Triterpenoids | Negative |
| 13 | Coumarins | Negative |
| 14 | Proteins and aminoacids | Positive |
| 15 | Steroids and phytosteroids | Positive |
Table 5: Result of phytochemical analysis
Phytochemical analysis of test seaweeds extract of dichloromethane showed the positive test for following contents like tannins, flavonoid, phenols, anthraquinones, alkaloids, quinines, proteins, aminoacids, Steroids and phytosteroids. When consider in aspect of antimicrobial activity phenolic are always in discussion as this compounds are auxiliary metabolites since they are not straightforwardly included in primary activities, for example, photosynthesis, cell division or reproduction processes of algae. This compound are known as aromatic ring due to its character with at least one hydroxyl attach to its structure and the antimicrobial activity is because of the modification of microbial cell porousness and the loss of intracellular macromolecules or by the obstruction with the membrane function and loss of cell integrity and finally leading to cell death (Abu-Ghannam et. al 2013). In case of flavonoid, they are hydroxylated phenolic compounds and are synthesized by seaweeds or plants in response to protect them self from microbial infection (R. A. Dixon et. al 1983). In consideration to as a dietary supplement, flavonoids are always in the list to have health-promoting properties due to presence of their high antioxidant capacity (N. C. Cook et. al 1996), (C. A. Rice-Evans et.al 1995). Following phytochemical test was found to be Coumarins, Coumarins, Triterpenoids, Cardiac glycosides, Carbohydrate, Anthraquinones, Saponin.
4.3 ANTIOXIDANT ACTIVITY:
4.3.1 Total phenolic content:
The total phenolic content of seaweed extracted using different solvent was measured using bio photometerically by Folin-Ciocalteau method. The results were expressed in terms of gallic acid equivalents (GAE). As shown in Table 6 and Figure 9, when compared Sargassum tenerrimum extract with different different solvents like Petroleum ether, Chloroform, Dichloromethane, Ethanol and Aqueous the phenolic content was found higher in dichloromethane extract which was about 1.3 mg per gram (GAE).
| EXTRACT SAMPLE | TOTAL PHENOLIC CONTENT mg/g (GAE) |
| CHLOROFORM | 0.6 |
| DICHLOROMETHANE | 1.3 |
| ETHANOL | 1 |
| PETROLEUM ETHER | 0.3 |
| AQUEOUS | 0.1 |
Table 6: Total phenolic content
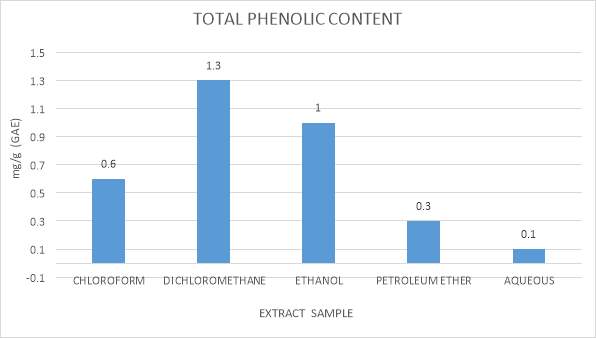
Fig 9: Total phenolic content graphical representation
4.3.2 Total flavonoid content:
Total flavonoid content of dried seaweeds of Sargassum tenerrimum extracted in different solvents is shown in Table 7 and Figure 10. Flavonoids are phenolics and are very important due to their biological and chemical activities, including some properties like an antioxidant and ability to scavenging free radical. if flavonoid content is considered for Sargassum tenerrimum in different solvents ethanol extract show maximum flavonoid contain 4.9 mg per gram and least flavonoid contain was observed in petroleum ether seaweed extract which was about 2.9 mg per gram.
| EXTRACT SAMPLE | TOTAL FLAVONOID CONTENT |
| CHLOROFORM | 3.6 |
| DICHLOROMETHANE | 3.3 |
| ETHANOL | 4.9 |
| PETROLEUM ETHER | 2.9 |
| AQUEOUS | 3.1 |
Table 7: Total Flavonoid Content
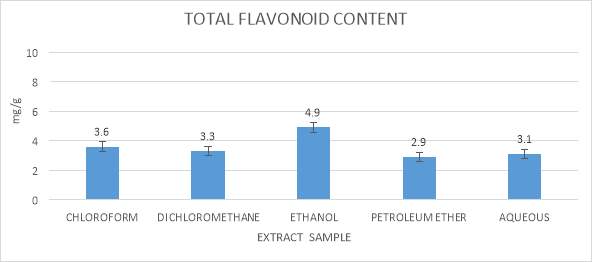
Fig 10: Total flavonoid contain graphical representation
4.3.3 Total Antioxidant activity:
Total antioxidant activity of Sargassum tenerrimum was carried out using phosphomolybdenum method. By caring out the activity using five different solvents the results are shown in Table 8 and Figure 11 indicates the total antioxidant activity of various extracts of Sargassum tenerrimum in different solvents. Estimation of total antioxidant activity of Sargassum tenerrimum indicated that activity was higher in ethanol extract which was about 25.5 mg per gram. Least activity was found in aqueous which was about 8.1 mg per gram.
| EXTRACT SAMPLE | TOTAL ANTIOXIDANT ACTIVITY |
| CHLOROFORM | 16.6 |
| DICHLOROMTHHANE | 12.9 |
| ETHANOL | 25.5 |
| PETROLEUM ETHER | 11.6 |
| AQUEOUS | 8.1 |
Table 8: Total antioxidant activity
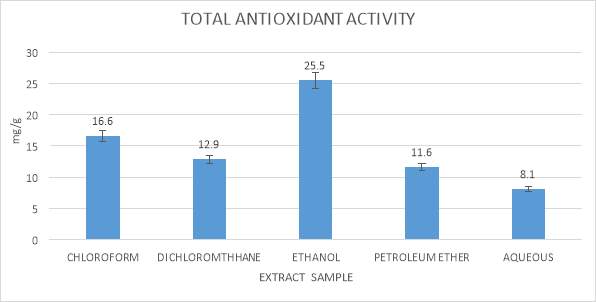
Fig 11: Total antioxidant activity graphical representation
4.3.4 DPPH radical scavenging:
DPPH has been used extensively as a stable free radical to evaluate reducing substances and is a useful reagent for investigating free radical scavenging activity of the components (Thoudam bhaigyabati 2011). DPPH radical scavenging result shown in (table 9) and (figure 12) indicates that ethanol extract of seaweed has maximum free radical scavenging property which was 40.3 percentage by this method and least scavenging property was shoed by acquous which was 30.1 percentage
| EXTRACT SAMPLE | FREE RADICAL SCAVENGING BY DPPH METHOD |
| CHLOROFORM | 38.6 |
| DICHLOROMTHHANE | 34.2 |
| ETHANOL | 40.3 |
| PETROLEUM ETHER | 38.7 |
| AQUEOUS | 30.1 |
Table 9: Free radical scavenging by DPPH method
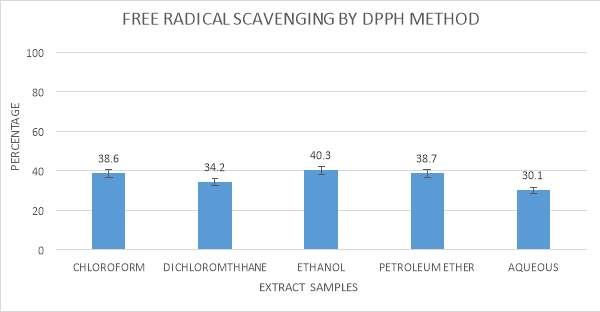
Fig 12: Free radical scavenging by DPPH method graphical representation
4.3.5 Hydrogen peroxide scavenging capacity:
Table 10 and Figure 13 indicates that ethanolic extracts of Sargassum tenerrimum exhibits a maximum H2O2 scavenging activity which was about 85.3 percentage. This is significantly higher than all the other solvent used. The lowest H2O2 scavenging property was observed in chloroform seaweed extract which was seen to be 54.6%
| EXTRACT SAMPLE | HYDROGEN PEROXIDE SCAVENGING |
| CHLOROFORM | 54.6 |
| DICHLOROMTHHANE | 62.2 |
| ETHANOL | 85.3 |
| PETROLEUM ETHER | 61.4 |
| AQUEOUS | 60.1 |
Table 10: Hydrogen peroxide scavenging activity
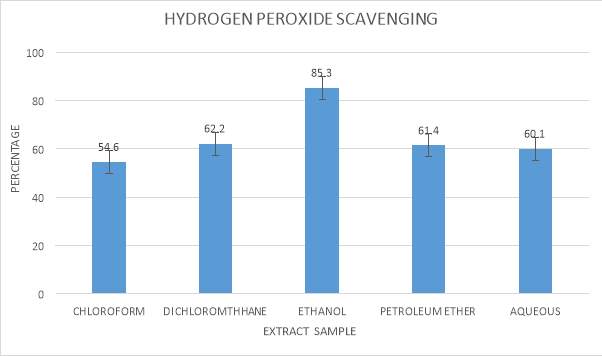
Fig 13: Hydrogen peroxide scavenging activity graphical representation
4.3.6 Hydroxyl radical scavenging assay:
Hydroxyl radical scavenging activity was investigated using the Fenton reaction and the results are shown in Figure 14 and Table 11. In this case, it was observed that the seaweed extract in solvent dichloromethane seen to have maximum hydroxyl radical scavenging activity which was about 55.2 percentage and chloroform was having least activity which was around 38.6 percentage.
| EXTRACT SAMPLE | HYDROXYL RADICAL SCAVENGING ACTIVITY PERCENTAGE |
| CHLOROFORM | 38.6 |
| DICHLOROMTHHANE | 55.2 |
| ETHANOL | 49.3 |
| PETROLEUM ETHER | 41.5 |
| AQUEOUS | 40.1 |
Table 11: Hydroxyl radical scavenging assay
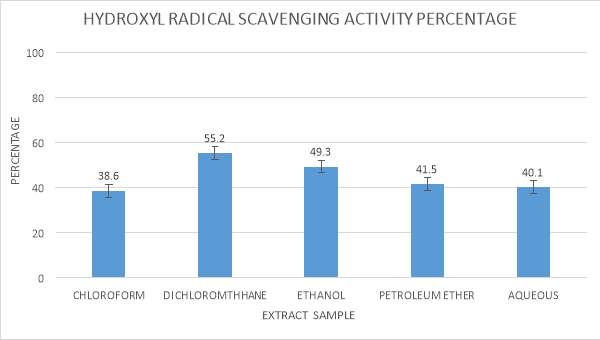
Fig 14: Hydroxyl radical scavenging assay graphical representation
4.3.7 ABTS radical scavenging assay:
The ABTS scavenging activity was determined in different extracts. The results are shown in Figure 15 and Table 12 ABTS scavenging activity was higher in ethanolic extract. In other extracts such as petroleum ether, Dichloromethane, chloroform and water extract activity is not high as ethanolic extract.
| EXTRACT SAMPLE | ANTIOXIDANT ACTIVITY BY RADICAL CATION (ABTS) |
| CHLOROFORM | 16.3 |
| DICHLOROMTHHANE | 14.1 |
| ETHANOL | 21.4 |
| PETROLEUM ETHER | 10.2 |
| AQUEOUS | 8.1 |
Table 12: ABTS radical scavenging assay
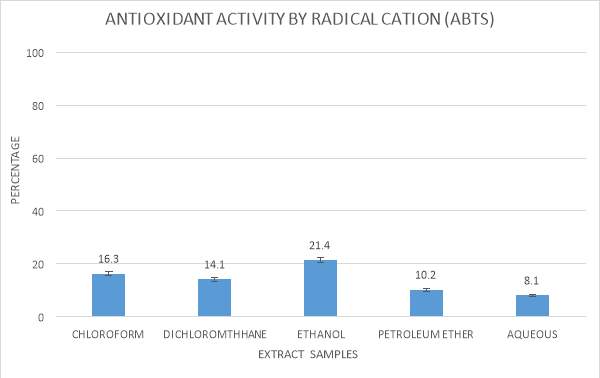
Fig 15: ABTS radical scavenging assay graphical representation
4.4 CHARACTERIZATION OF ACTIVE COMPOUNDS:
4.4.1 GC-MS analysis:
Gas Chromatography–Mass Spectrometry (GC–MS) has been utilized widely to recognize the structures and the components of various phytoconstituents in plant extract and other biological samples with great achievement (Prasain et al., 2004). GC–MS is a solid strategy to distinguish the constituents of unpredictable matter such as long-chain or branched chain hydrocarbons, alcohols acids and esters (Anjali et al., 2009). This recognizable proof was carried out by correlation of their mass spectra on both columns with phytochemical and ethnobotanical databases libraries or with mass spectra from the other present literature (Jennings and Shibamoto, 1980) and home-made library. Various phytocomponents from different seaweeds were seen in GC –MS. Out of the component detected, five component was recorded earlier for having anti-microbial activity. The outcomes are detailed in a table with their peaks obtained in graph.
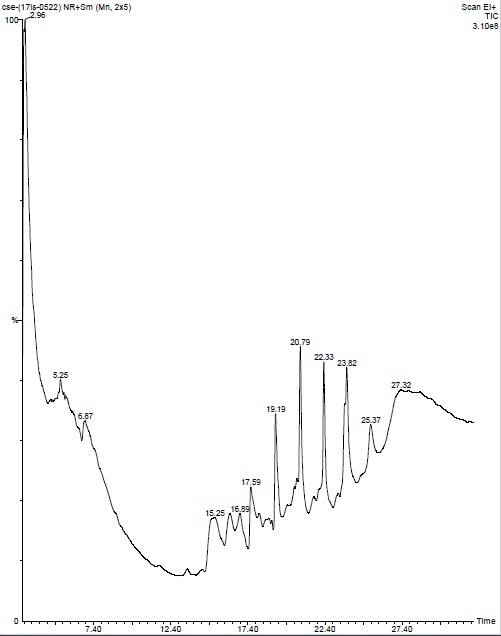
Fig 16: GC-MS of chloroform seaweeds extract
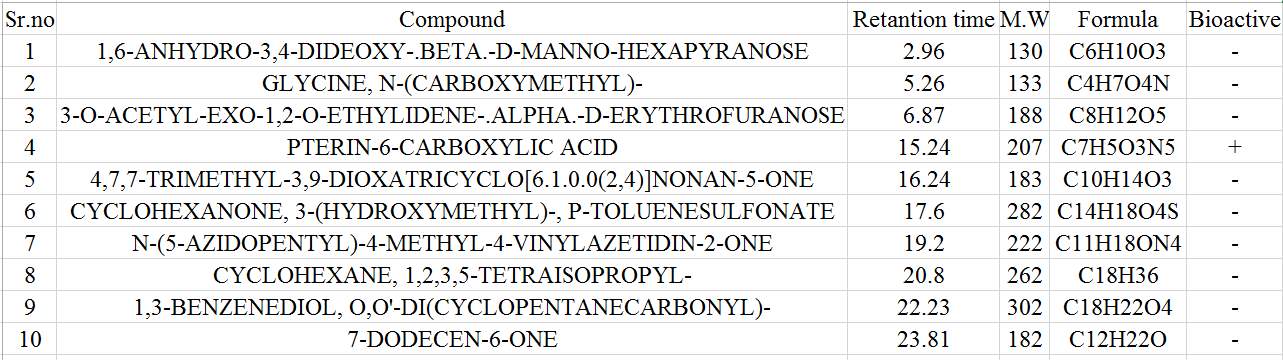
Table 13: GC-MS compound of chloroform seaweeds extract
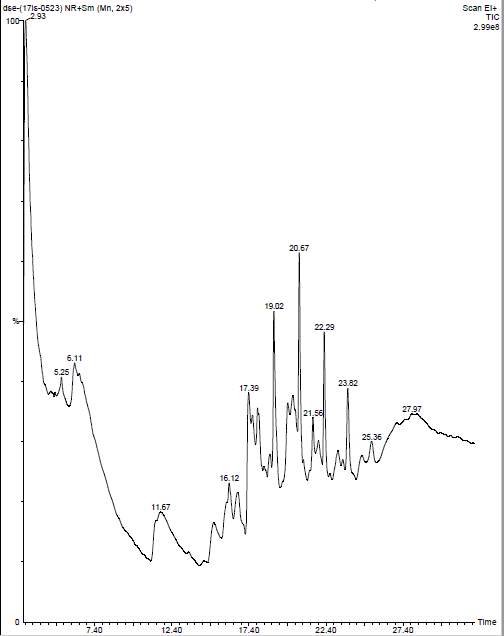
Fig 17: GC-MS of Dichloromethane seaweeds extract
Table 14: GC-MS compounds of Dichloromethane seaweeds extract
Fig 18: GC-MS of ethanol seaweeds extract

Table 15: GC-MS compounds of ethanol seaweeds extract
Fig 19: GC-MS of petroleum ether seaweeds extract

Table 16: GC-MS compounds of petroleum ether seaweeds extract
4.4.2 HPLC analysis:
HPLC analysis was carried out for three solvents of seaweeds extract namely chloroform, dichloromethane and ethanol using standard procedure and the major peaks were studied and identified for the presence of compound that denotes it which is represented in (table 15)
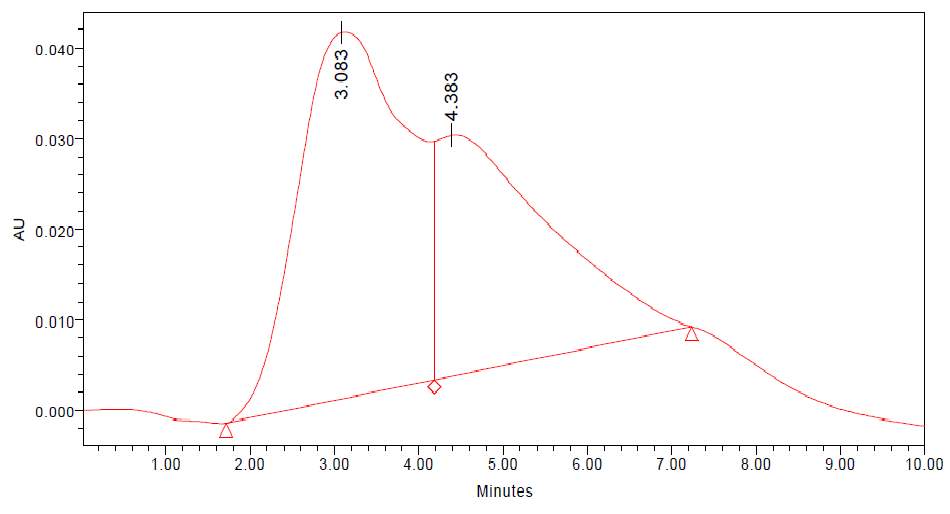
Fig 20: HPLC of chloroform seaweeds extract
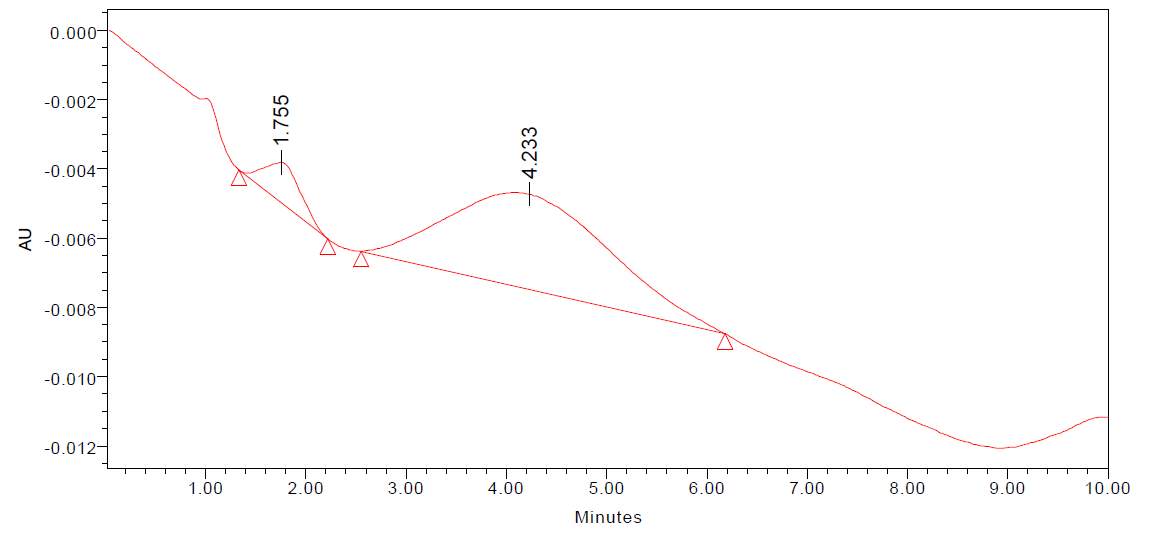
Fig 21: HPLC of dichloromethane seaweeds extract
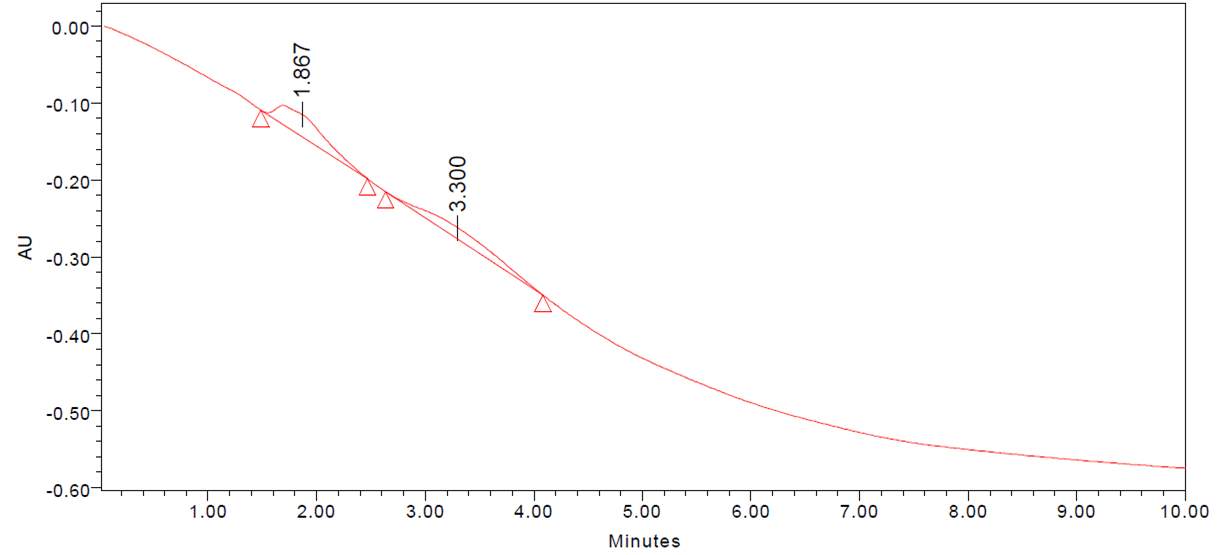
Fig 22: HPLC of petroleum ether seaweeds extract
| PEAK VALUE | COMPOUND |
| 3.083 | Gallic Acid |
| 4.383 | Catechin |
| 4.235 | Catechin |
| 3.300 | Quercitin |
Table 17: Results of HPLC
4.4.3 FT-IR analysis:
FT-IR was performed on seaweed extract of different solvents with crude and chromatography (purified). The functional groups present were identified alcohol, alkane, carbonyl, amine, aromatic, ester, ether, carboxylic acids, aldehydes, carboxylates and alkene. The resultant peak frequencies were identified from the standard peaks.
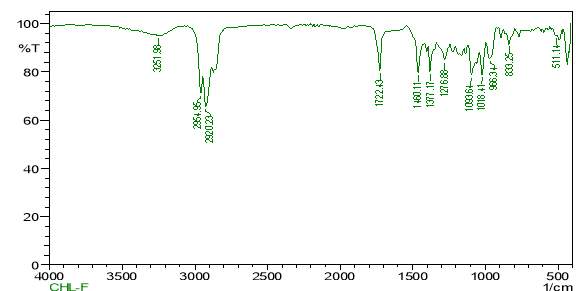
Fig 23: FT-IR of Chloroform crude extract
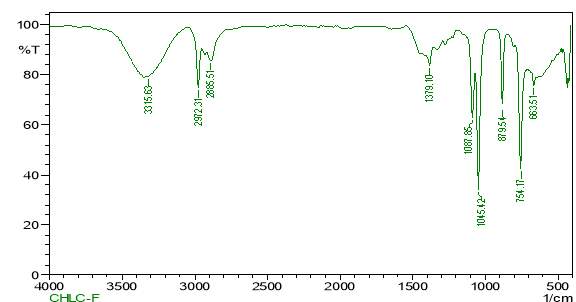
Fig 24: FT-IR of chloroform purified extract
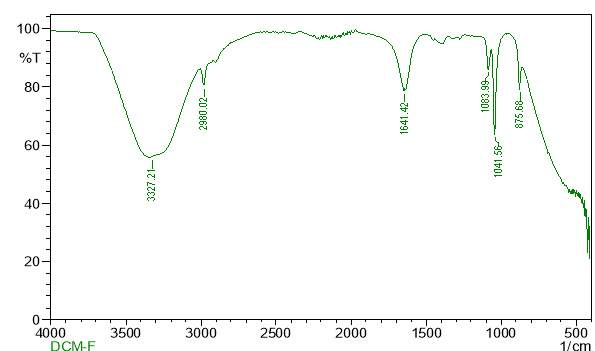
Fig 25: FT-IR of dichloromethane crude extract
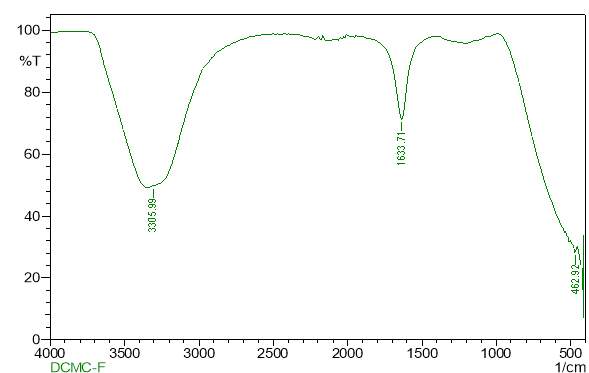
Fig 26: FT-IR of dichloromethane purified extract
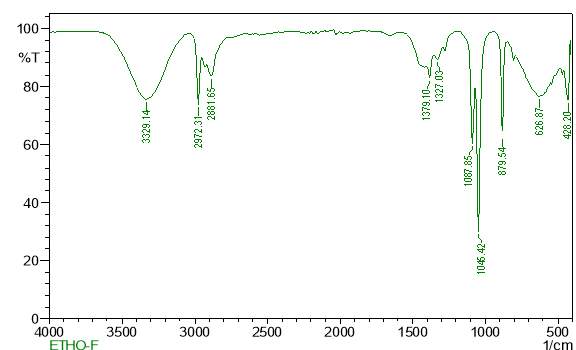
Fig 27: FT-IR of ethanol crude extract
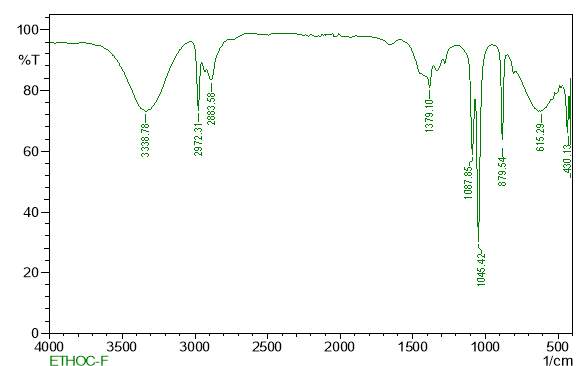
Fig 28: FT-IR of ethanol purified extract
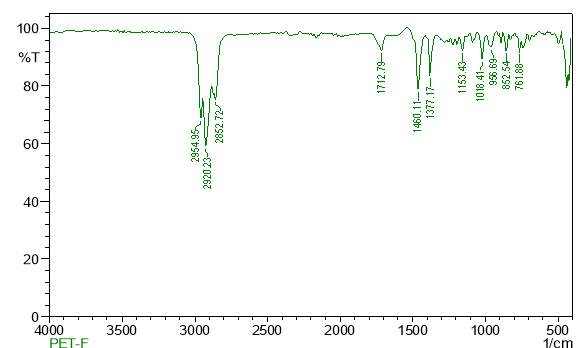
Fig 29: FT-IR of petroleum ether crude extract
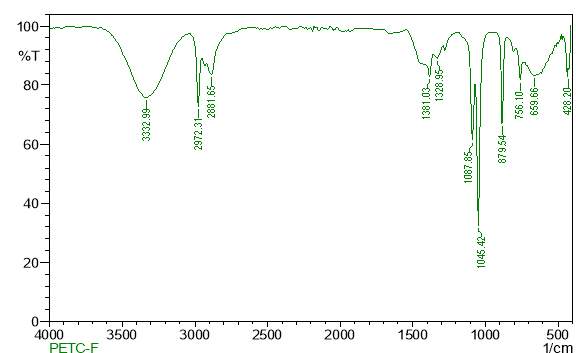
Fig 30: FT-IR of petroleum ether purified extract
4.4.4 FT-RAMAN analysis:
FT-Raman was performed on seaweed extract of two solvents with crude and chromatography (purified). The functional groups present were identified alcohol, alkane, carbonyl, amine, aromatic, ester, ether, carboxylic acids, aldehydes, carboxylates and alkene. The resultant peak frequencies were identified from the standard peaks.
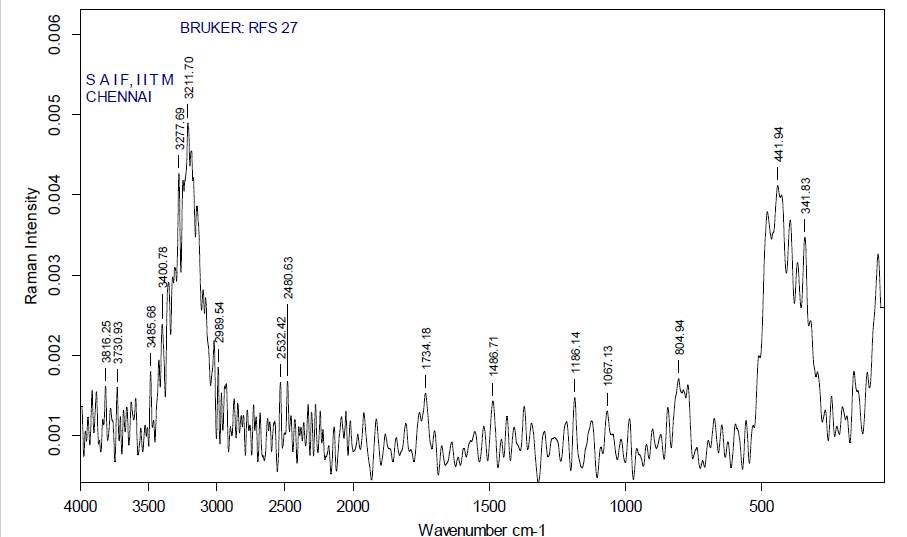
Fig 31: FT-Raman of dichloromethane crude extract
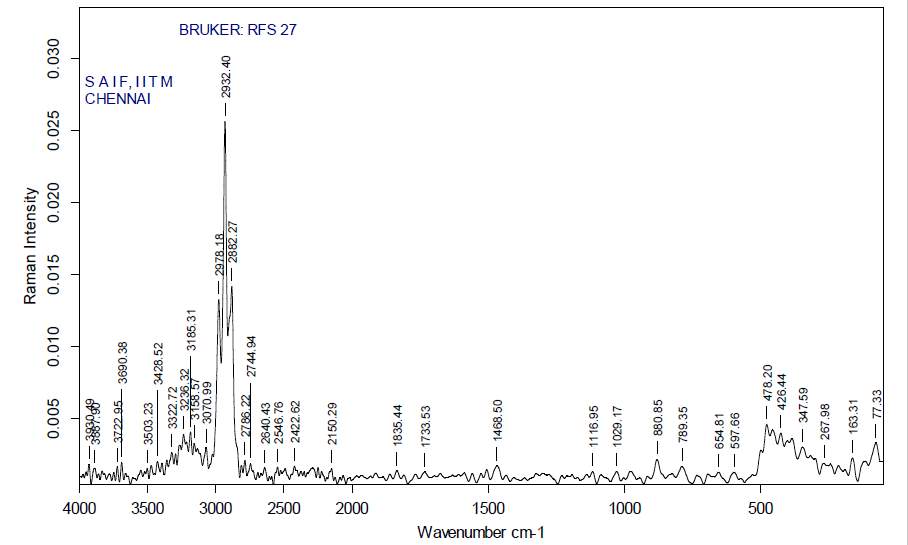
Fig 32: FT-Raman of dichloromethane purified extract
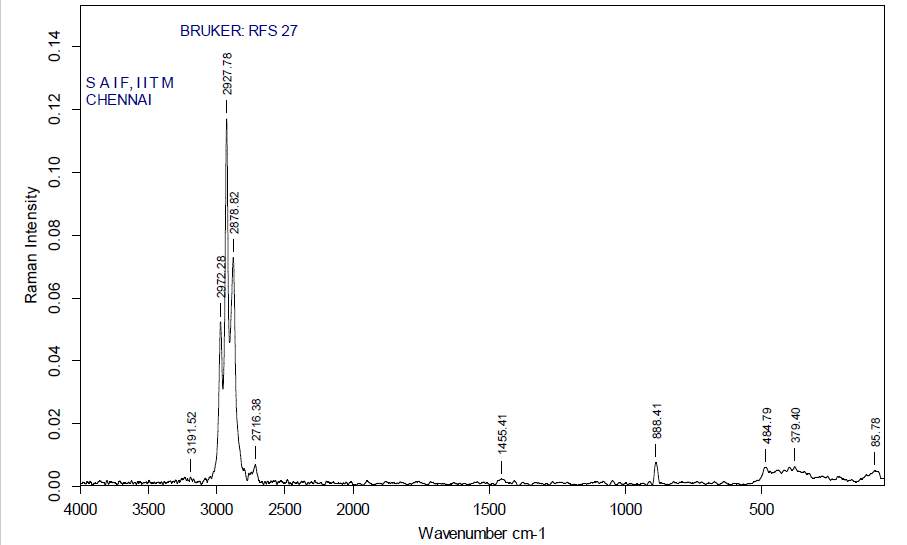
Fig 33: FT-Raman of ethanol crude extract
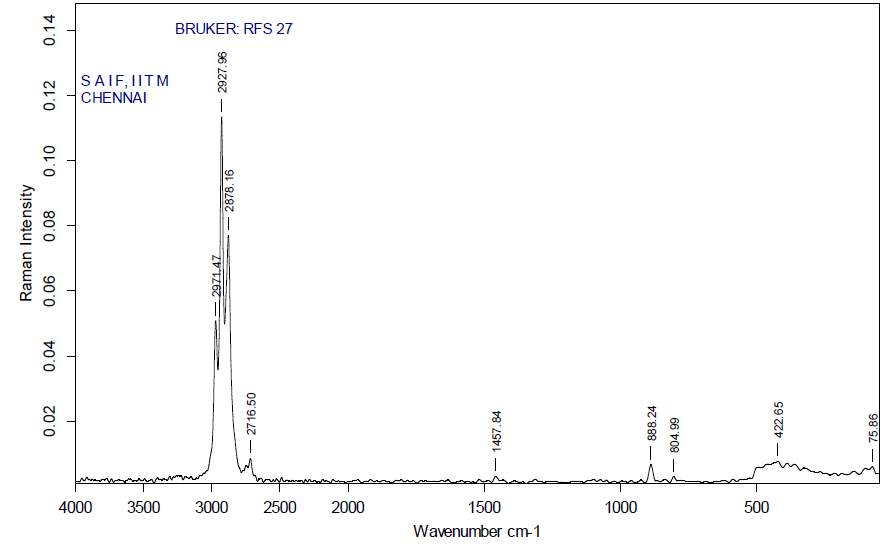
Fig 34: FT-Raman of ethanol purified extract
4.5 INVITRO STUDY OF ANTIBACTERIAL ACTIVITY:
4.5.1 Well diffusion Method:
Well diffusion method was carried out for five different seaweed extract. In this study the anti-bacterial activity of V. parahaemolyticus against Sargassum tenerrimum. The results revealted that a zone of inhibition of 12 mm in 50 microliters of dichloromethane was observed in the extarct loaded well. Dichloromethane extract showed good zone of inhibition compared to other extract No zone of inhibition was seen in control well loaded with dichloromethane. The control did not exhibit any zone of inhibition. It can be reasoned that Sargassum tenerrimum exhibits antibacterial activity against V. parahaemolyticus previous work on the antibacterial activities of algae provides evidence of the importance of some brown seaweed against pathogens. In the present work, Sargassum tenerrimum was found to exhibit potent antibacterial activity against V. parahaemolyticus. The dichloromethane extracts of Sargassum tenerrimum were found to be the most effective against the pathogen tested.
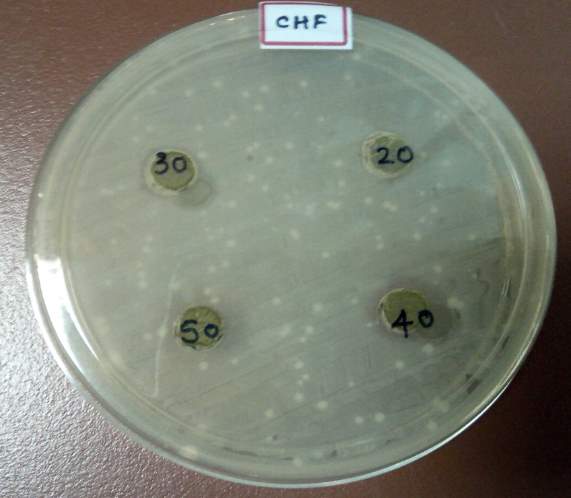
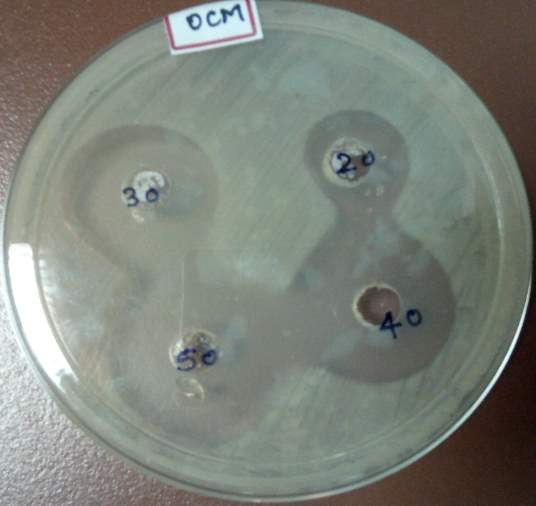
Fig 35: well diffusion Chloroform extract Fig 36: well diffusion Dichloromethane extract
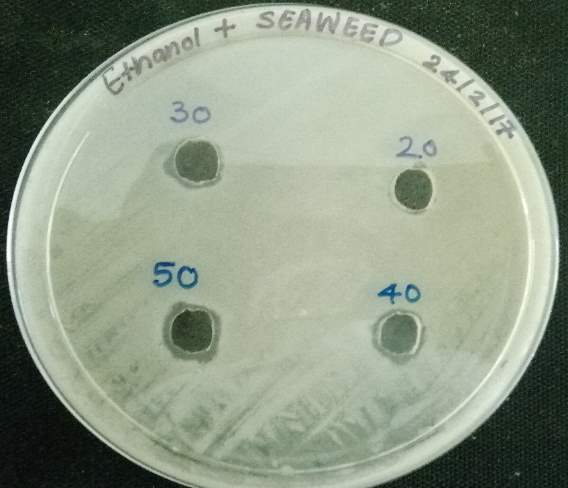
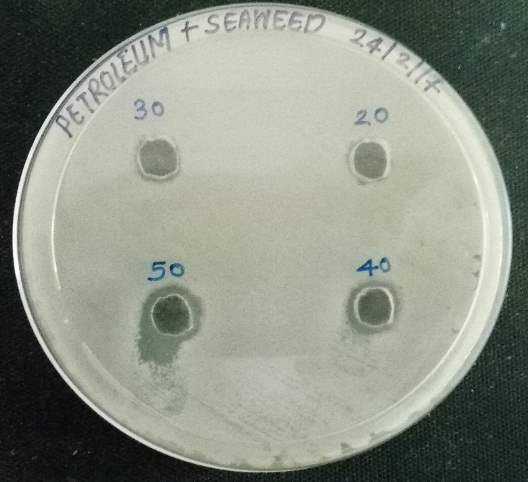
Fig 37: well diffusionEthanol extract Fig 38: well diffusion Petroleum ether extract

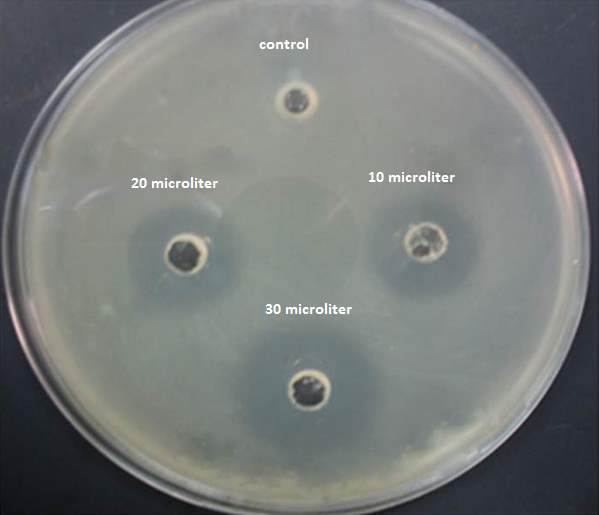
Fig 39: well diffusion water extract Fig 40: control with Dichloromethane extract
4.5.2 Disc diffusion method:
The results revealed that the extract loaded disc had a zone of inhibition of 10mm for dichloromethane and other extract to show the slight zone of inhibition. Heavy antibiotic use in aquaculture needs to be reduced and replaced with alternative processes for treating diseases in prawn to avoid the emergence of antibiotic resistance in pathogenic and environmental bacteria (Sørum and L’Abée-Lund, 2002; Cabello, 2006). Natural substances like thyme oil, clove oil and pine oil were used as alternative bio-herbicides and bio-pesticides in ecological agriculture (Verschwele, 2005; Perez and Lewis, 2006). Similarly, the herbal plants and algae may be used as potential and promising sources of pharmaceutical agents against pathogen in aquaculture. Resistance to the antimicrobial agents used in aquaculture has increased in many countries in recent years. A wide range of antimicrobial compounds are now being used in aquaculture. Attention has been drawn to the possible development of antibiotic resistant bacteria because the emergence of resistant bacteria has been repeatedly demonstrated in a variety of environments and media: hospital and barnyard, man and animal. The present study revealed that the seaweeds extract can be used for treating the bacterial infection in aquaculture.
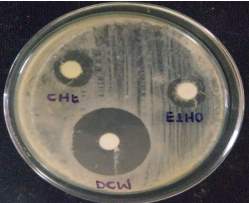
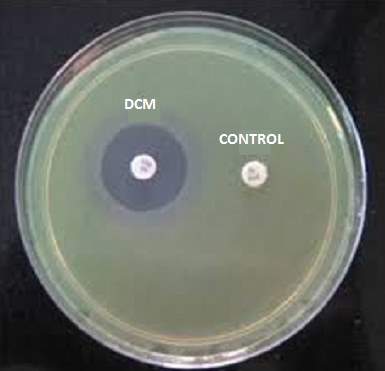
Fig 41: Disc diffusion test Fig 42: Controls
4.5.3 Minimum inhibitory concentration:
The MIC of Sargassum tenerrimum was determined by the tube dilution technique. Sargassum tenerrimum was found to be effective in controlling the Vibrioparahemoleticusat20 ppm level in dichloromethane. Hence the minimum inhibitory concentration was found to be 20 ppm. For other extract MIC was abserved in range of 40 ppm to 60 ppm the results are presented in table 18. In the following table (+++) denotes very high growth, (++) denotes high growth, (+) denotes less growth, ( [+] ) denotes very less growth, (-) denotes no growth of V. parahaemolyticus.
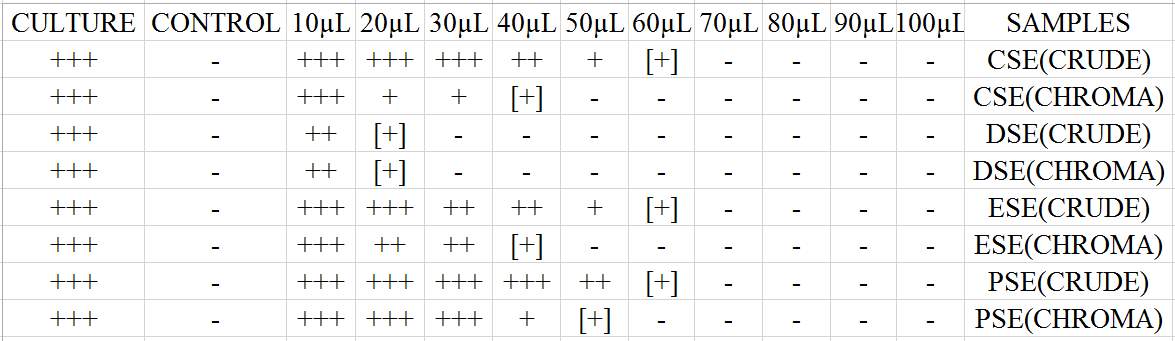
Table 18: Minimum inhibitory concentration
4.5.4 Growth kinetic assay:
Growth kinetic study of all the seaweed extract was carried out. In this study it was observed that dichloromethane dost not allow the growth of bacterial culture and inhibits the multiplication of the bacterial culture. Bacterial growth phases like lag, log, stationary and decline phases are not seen in flask loaded with the seaweed extract. The flask with the control shows proper growth phases.
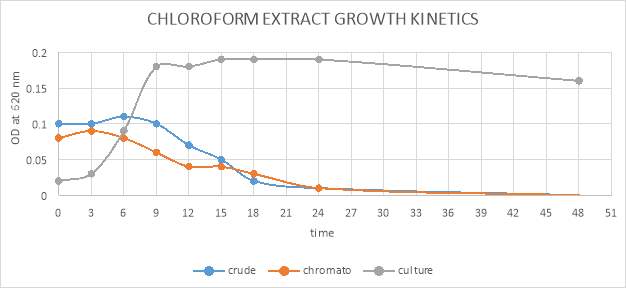
Fig 43: growth kinetic of chloroform crude and purified extract graphical representation. 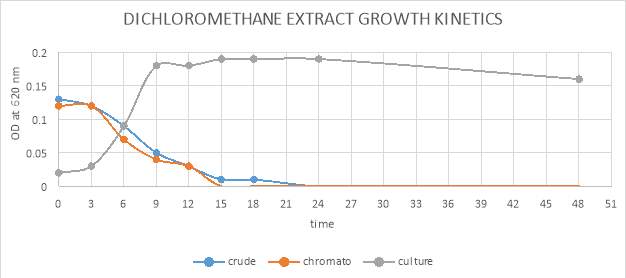
Fig 44: growth kinetic of dichloromethane crude and purified extract graphical representation.
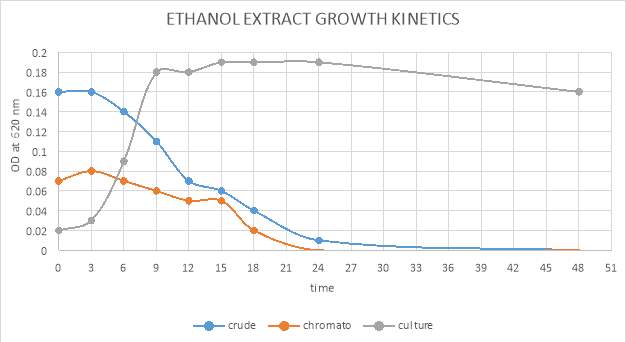
Fig 45: growth kinetic of ethanol crude and purified extract graphical representation.
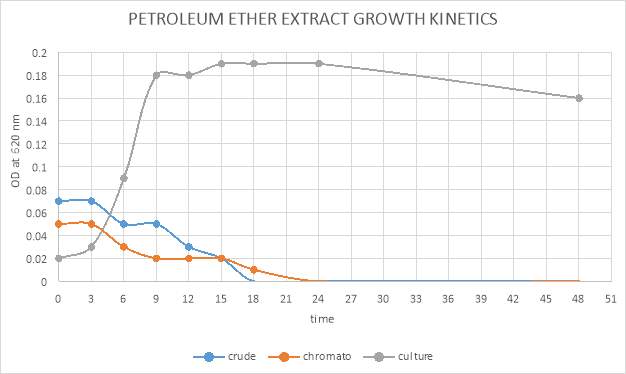
Fig 46: growth kinetic of petroleum ether crude and purified extract graphical representation.
4.6. EXTERIMENTAL PATHOGENICITY OF BACTERIAL CULTURE:
4.6.1 Experimental pathogenicity intramuscular and immersion:
The pathogenecity of M. rosenbergii to V. parahaemolyticus was confirmed by intramuscular injection and by water bath method. The clinical symptoms were observed such as lethargy, reddish and green colouration on the body scales was seen. The highest concentration of V.parahemoleticus caused 100% mortality within 48 hrs of post inoculation in imtramuscular and in immerstion too. However the lower concentrations of V. parahaemolyticus caused 0 % and 33% mortality, after 120 and 96 h respectively in both immersion and intramuscular. The LD50 value of V. parahaemolyticusfor intramuscular route was found to be 2.4 × 10-4 at 120 hrs. Of post injection. The LC50 valve for immersion was found to be 1.3 x 10-4 at 120 hours. The mechanisim of infection may be a combination of invasive and toxic pathways. A major setback in aquaculture is the sudden outbreak of diseases, especially those caused by Vibrio spp, which are considered a significant problem to the development of a sector with severe economic losses worldwide. Vibrio harveyi, Vibrio parahemoleticus and Vibrio anguillarum are most frequently isolated marine Vibriospecies as reported by (Arias et al., 1999; Pujalte et al., 1999) . (Rajesh Kumar et al., 2008) Reported that once the pathogen colonized the intestine, they penetrate the intestinal wall and cause a systemic infection resulting in disease and death. Disease caused by V. parahaemolyticus is a major problem in sea bass farming worldwide. The results are shown in figure 46 of infected shrimps and control in fig 45.


Fig 47: Control shrimp Fig 48: Infected shrimp
4.6.2 Post larvae toxicity:
Post larvae toxicity bioassay of purified seaweed extracts was performed for the optimum exposure in Artemia salina with different concentrations from 20-100 µg/ml. The results of this toxicity study showed the maximum 100 µg/ml resulted only 30% accumulated mortality at the 120th hour of the post-treatment period, as shown in figure 47. Whereas, in lowest concentration there was no significant mortality was found. This Ichthyotoxic study in rohu fish revealed that the purified seaweed extract of C. linum extract does not cause harm to the fishes exposed to the maximum concentration. Similarly (Ayesha et al., 2010) studied the cytotoxic behavior of nine different seaweeds, extracted using different solvents for their optimum toxicity exposure level in brine shrimps. Their study suggested and classified the degrees of toxicity levels for the cytotoxic experiments, namely nontoxic, mild toxic, highly toxic. Mortality rate between 25- 50% is nontoxic, above 50% to less than75% is mild toxic and above 75% is highly toxic. The present exhibit the toxicity bioassay of C. linum found to be nontoxic to the Artemia cysts according to the report of (Ayesha et al., 2010).
Fig 49: post larvae toxicity assay graphical representation
4.7 TREATMENT AND FORMULATION OF BEADS
4.7.1 Treatment using intramuscular injection:
The study completed showed that the antibacterial activity in seaweed extracts affected the microorganisms which were one that causes disease in M. rosenbergii. In this study seaweeds extract are used in two forms one with combination of ethanol and petroleum ether extract and one was only carried out with dichloromethane. The new freshwater prawns were sound and no indications of manifestations. In the extract from seaweed compound was helpful in treating the illness and 100% survival was noted in combination treatment and one with only dichloromethane. India is rich in therapeutic algae and plants. An extensive bit of the total populace, particularly in developing countries depend on upon the conventional method of medication for different forms of the disease. Present day science has not possessed the capacity to supplant a large portion of them. Because of the aimless utilization of antimicrobial medications microorganisms have created drug resistant to numerous anti-infection agents. This has made massive clinical issues in the treatment of irresistible illnesses (Davis, 1994).

Fig 50: Treated shrimp
4.7.2 Encapsulation of crude compound and treatment using encapsulated beads:
Encapsulation of crude compounds was carried out with two extract namely ethanol and dichloromethane using casein and xanthum gum formulation which is a biologically significant process which entrap the proteins and other biomolecules in the interfacial complex system for the stability of the carbohydrate polymer matrix (Reddy et al.2011; Shahidi and Han, 1993). Generally, the casein product derivatives are huge source that are encompassed with proteins. The beads incorporated with this seaweed extract made up with different solvent are used in the aquatic environment to ascertain the encapsulation efficiency (Hogan et al.2001). In the present study the two crude compounds were encapsulated in the form of beads which can be used in feed treatment. Similarly (Dehkharghanian et al.2009) reported that Na) caseinate was used for the encapsulation of green tea Polyphenol. This agrees with our method of incorporating the compounds of seaweeds into Na) caseinate beads for use as a novel feed to control various bacterial infection. Encapsulated various natural product extracts have been used for different antimicrobial treatment applications and widely was in aquaculture (Reverter et al.2014). The results revelated that the fresh water prawn fed with the prepared beads of ethonal and dichloromethane was able to survive for a period of more than 20 days (Fig.49 and Fig.50).


Fig 51: Circular beads formation in 1N HCl Fig 52: Dried beads
4.7.3 SEM analysis of beads:
The SEM micrograph showed the result with spherical shape morphology of the microencapsulated of seaweed beads. Considerable mean diameter was approximately found to be around 100µm. No visible cracks or pores were seen on the outer surface of the encapsulated beads. The results are shown in (Fig 51).
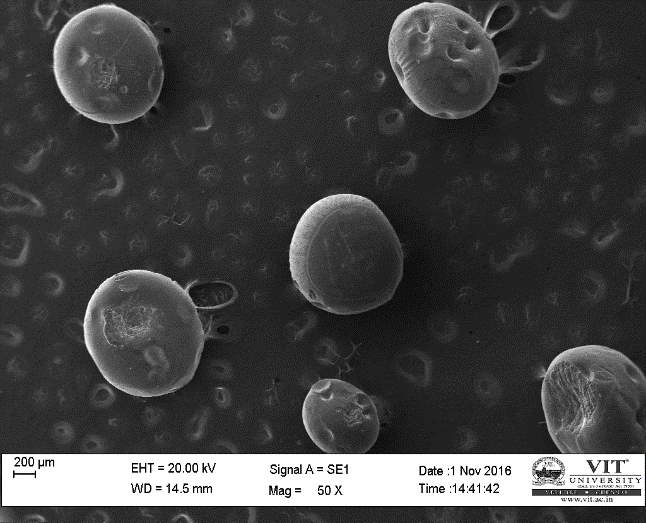
Fig 53: SEM analysis of encapsulated beads
4.8 Histopathology investigation:
Histological investigation was carried out of organs such as gill, hepatopancreas, and muscle of control and infected shrimps they were investigated structural changes in body organs due to vibrio infection. In case of muscle the vacuolation was seen in infected shrimp. Histological studies were carried out on the gill, muscle and hepatopancreas of ADS-affected prawn by Pillai et al. (2005). Exudation in the gills, muscle and rarely in the hepatopancreas of severely affected prawn was noted, but detailed histological study was not carried out. Sahoo et al. (2008) have not observed any significant pathological changes in muscle tissue or hepatopancreas of ADS-affected prawn. The extensive vacuolation due to accumulation of fat was observed in the hepatopancreas of infected prawn and not in control. In case of gills the gill lamellar cells were shrunken with pyknotic nuclei. Some lamellar cells showed extensive vacuolation and also found structural distortation of lamellar. In the present study as observed in Vibrio – infected larvae of American oyster Crassostrea virginica. Similarly Burke et al (1981) have observed high vacuolation in the hepatopancreas of Vibrio – infected
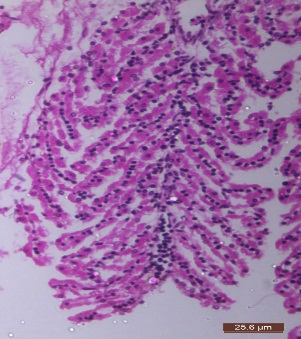
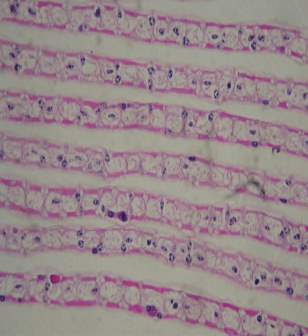
Fig 54: Infected gills Fig 55: Control gills
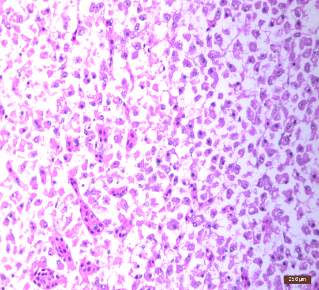
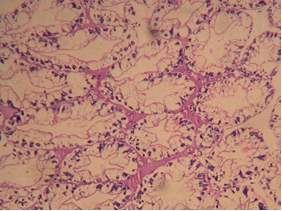
Fig 56: Infected Hepatopancreas Fig 57: Control Hepatopancreas
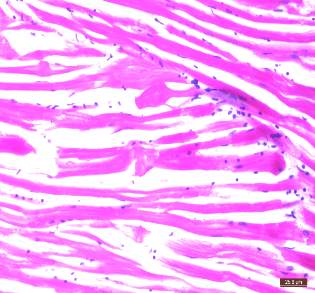
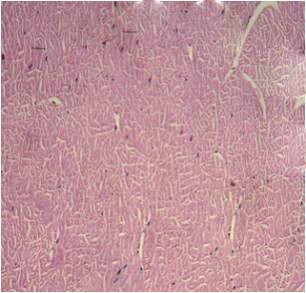
Fig 58: Infected tissue Fig 59: Control tissue
4.9 COMET ASSAY:
Comet assay was carried out for control, infected and treated shrimp. Results acquired from comet showed that in control and treated the cell observed under fluorescence microscope where circular and no tail was observed. In case of infected the cell under fluorescence was observed to tailed like structure. The tail length in the infected was around 4.52 micrometers.

Fig 60: Infected cell by comet assay Fig 61: Control cell by comet assay
5. CONCLUSION:
Marine brown algae are known to be produces of largest biomass in the aquatic environment and they are also represent for its highly diverse and unique bioactive potential compounds present in them. Many of the substances obtained from seaweed, such as alginates, carrageenan and agar have been serving to mankind since many years in traditional medicine, pharmacology and food. Other compounds that serves as bacteriostatic or antibacterial, antiviral, antitumor, anti-inflammatory and antifouling activities that have been in a great demand due to its activity mentioned above. Therefore, seaweed could provide promising bioactive that can be used in the treatment of various diseases in human as well as animals and also serve as a new option for antimicrobial agents to replace synthetic antibacterial agents used in agriculture and in the food industry and aquaculture. This study concludes that the seaweeds due to their property like antioxidant activity and antibacterial activity the can be used in various field in order to bring novel change in field of developing new neutraceuticals to fight against various infection in a natural way.
6. REFERENCES:
Abu-Ghannam, N.; Rajauria, G. Antimicrobial activity of compounds isolated from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 287–306.
Alderman, D.J. and C. Michel. 1992. Chemotherapy in Aquaculture Today. In: Chemotherapy in Aquaculture from Theory to Reality (ed. by C. Michel and D.J. Alderman), Office • 293 • International Des Epizooties, Paris. pp 3-4.
Amin Ismail & Tan Siew Hong (2002).Antioxidant Activity of Selected Commercial Seaweeds Department of Nutrition and Health Sciences, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia Mal J Nutr 8(2): 167-177.
Anjali Ruikar, Rasika Toranel, Amruta Tambe, Vedavati Puranik, and Nirmala Deshpande, 2009. GC – MS study of a steam volatile matter from Mimusops elengi. Int J. Chem. Tec. Res, Vol.1, 158-161
Arias CR, Macian MC, Aznar R, Garay E, Pujalte MJ (1999) Low incidence of Vibrio vulnificus among Vibrio isolates from seawater and shellfish of the western Mediterranean coast. J Appl Microbiol 86: 125-134.
Bhaskar N and Miyashita k., 2005 “Lipid composition of Padinatetratomatica (Dictyotales, Phaeophyta) brown seaweed of the west coast of India”. Indian Journal of Fish. 52, 263-268.
Burke, V., Gracey, M., Robinson, J., Peck, D., Beaman, J., and Bundell, C. 1981. The microbiology of childhood gastroenteritis: Aeromonas species and other infective agents. Journal of Infectious Diseases, Vol. 148, 68-74.
C. A. Rice-Evans, N. J. Miller, P. G. Bolwell, P. M. Broamley, and J. B. Pridham, “The relative antioxidant activities of plant-derived polyphenolic flavonoids,” Free Radical Research, vol. 22, no. 4, pp. 375–383, 1995.
Cabello, F.C., 2006. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environmental Microbiology, Vol. 8(7), 1137-1144
Cardozo, K.H.M., Guaratini, T, Barros M.P, Falco V.R., Tonon A.P., Lopes N.P., Campos,s., Torres,M.A., Souza A.o., Colepicolo,p and Pinto, 2007. “Metabolites from algae with economical important’.Comparative Biochemistry and Physiology-part- C: Toxicology and Pharmacology.146; 60-78.
Cavallo, R.A.; Acquaviva, M.I.; Stabili, L.; Cecere, E.; Petrocelli, A.; Narracci, M. Antibacterial activity of marine macroalgae against fish pathogenic Vibrio species. Cent. Eur. J. Biol. 2013, 8, 646–653.
Changyi, L., L. Liangmu and W. Hehai. 1997. Composition of fatty acids in mangrove leaves and their worth as natural resources. Xiamen Danue Xuebao, Ziran Kexueban 36: 454-459
Choudhury, S., P. Pattnaik, A. Sree, M. Bapuji and S.C. Mukherjee. 2002. Effect of soft coral, gorgonian and hydroid extracts on growth inhibition of fish pathogenic bacteria. Journal of Aquaculture in the Tropics 17: 319-323.
Choudhury, S., P. Pattnaik, A. Sree, M. Bapuji and S.C. Mukherjee. 2003. Antibacterial activity of sponge extracts against fish pathogens. Aquaculture Research 34: 1075-1077.
Cooper, S., A. Battat, P. Marot and M. Sylvester. 1983. Production of antibacterial activities by two bacillariophyceae grown in dialysis culture. Canadian Journal of Microbiology 29: 338-341.
El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25.
Findlay, J.A. and A.D. Patil. 1984. Antibacterial constituents of the diatom Navicula delognei. Journal of Natural Products. 47: 815-818
Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609.
Gupta, S.; Cox, S.; Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N. Growth inhibition of common food spoilage and pathogenic microorganisms in the presence of brown seaweed extracts. Food Bioprocess Technol. 2012, 5, 1907–1916.
Harborne JB, 1973. Phytochemical Methods, 3rd Ed. Chapman and Hall, London. pp. 41 -48.
Hogan, S. A., McNamee, B. F., O’Riordan, E. D., & O ‘Sullivan, M. (2001) microencapsulating properties of sodium caseinate. J. Agric. Food Chem 49(4), 1934)1938.
Imran Khan, Surya Rao Srikakolupu, Surekha Darsipudi, Srujana Divya Gotteti and Hemasundara Amaranadh.Ch, 2010, Phytochemical studies and screening of leaf extracts of Azadirachta indica for its anti-microbial activity against dental pathogens, Archives of Applied Science Research, 2 (2), 246-250.
Jennings, W.G., and Shibamoto, T., 1980. Quantitative Analysis of Flavor and Fragrance Volatiles by Glass Ccapillary G.C. Academic Press, New York.
Kavita, K.; Singh, V.K.; Jha, B. 24-branched delta 5 sterols from Laurencia papillosa red seaweed with antibacterial activity against human pathogenic bacteria. Microbiol. Res. 2014, 169, 301–306.
Kellam, S.J., R.J.P. Cannell, A.M. Owsianka and J.M. Walker. 1988. Results of a large scale screening programme to detect antifungal activity from marine and freshwater microalgae in laboratory culture. British Phycological Journal. 23: 45-47
Krish, S.; Das, A. In-vitro bioactivity of marine seaweed, Cladophora rupestris. Int. J. Pharm. Biol. Sci. 2014, 5, 898–908.
Lawson L.D., Garlic a review of its medicinal effects and indicated active compounds. 1998. In: Lawson L.D, Bauer R, eds. Phytomedicines of Europe: their chemistry and biological activity. Washington DC: American Chemical Society, 69.
Mahasneh, I., M. Jamal, M. Kashasneh and M. Ziodeh. 1995. Antibiotic activity of marine algae against multiantibiotic resistant bacteria. Microbio 83: 23-26.
Microbiology, Vol. 78, 43–56.
N. C. Cook and S. Samman, “Review: flavonoids-chemistry, metabolism, cardioprotective effects and dietary sources,” Journal of Nutritional Biochemistry, vol. 7, no. 2, pp. 66–76, 1996.
Olabinri B. M., Adepoju E. A., Zainab A. A. and Ahmed A. A., Phytochemical profiling of phytoconstituents of grape, Jatropha curcas and Neem (Azadirachta indica) extracts, 6(2), 17-23, 2014
Pai S.T., Platt, M.V., 1992. Antifungal effects of Allium sativum (garlic) extract against the Aspergillus species involved in otomycosis. Clinical Microbiology, Vol. 30, 2881-6.
Premalatha.M, Dhasarathan P, Theriappan P 2011. Phytochemical characterization and antimicrobial efficiency of seaweed sample Ulva fasciata and Chaetomorpha antennina. International Journal of Pharma and Bio Sciences, 2(1) ISSN 0975- 6299.
Premnathan, M., H. Nakashima, K. Kathiresan, N. Rajendran and N. Yamamoto. 1996. In vitro anti- human immunodeficiency virus activity of mangrove plants. Indian Journal of Medical Research. 103: 278-281.
Pujalte MJ, Ortigosa M, Macian MC, Garay E (1999) Aerobic and facultative anaerobic heterotrophic bacteria associated to Mediterranean oysters and seawater. Int Microbiol 2: 259-266.
R. A. Dixon, P. M. Dey, and C. J. Lamb, “Phytoalexins: enzymology and molecular biology,” Advances in Enzymology and Related Areas of Molecular Biology, vol. 55, pp. 1–136, 1983.
Reddy, B. C., Noor, A., Sarada, N. C., & Vijayalakshmi, M. A. 2011) Antioxidant properties of Cordyline terminalis (L.) Kunth and yristica fragrans Houtt. encapsulated separately into casein beads. Curr Sci 101(3), 416)420. Arc pincode 121
Reuter, H.D., Koch, H.P., and Lawson, L.D., 1996. Therapeutic effects and applications of garlic and its preparations. In: Koch HP, Lawson LD, eds. Garlic. The science and therapeutic application of Allium sativum L. and related species. Baltimore: Williams and Wilkins, 135-212.
Reverter, M., Bontemps, N., Lecchini, D., Banaigs, B., & Sasal, P. (2014) Use of plant extracts in fish aquaculture as n alternative to chemotherapy: current status and future perspectives. Aquacult 433, 50)61
S.Rajesh Kumar, V.P. Ishaq Ahmed, V. Parameswaran, R. Sudhakaran, V. Sarath Babu, A.S. Sahul Hameed Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from Vibrio Listonella) anguillarum Fish & Shellfish Immunology (2008) 25, 47e56
Sachithananthan, K. and A. Sivapalan. 1975. Antibacterial properties of some marine algae of Sri Lanka. Bulletin of Fisheries Research Station, Sri Lanka. 26: 5-9.
Sadasivam S, Manickam A (1996). Biochemical Methods for Agricultural Sciences, New Age International (P) Ltd., New Delhi, India, 1-97.
Sahoo, S.K., Giri, S.S., Chandra, S., and Mohapatra 2008. Evaluation of breeding performance of Asian Clarias batrachus at different dose of HCG and latency combinations. Turkish Journal of fisheries and aquatic sciences, Vol. 8, 249 -251.
Shahidi, F., & Han, X. Q. (1993) Encapsulation of food ingredients. Crit Rev Food Sci Nutr33 (6), 501)547
Smith, P., M.P. Hiney and O.B. Samuelsen. 1994. Bacterial resistance to antimicrobial agents used in fish farming. Annual Review of Fish Diseases 4: 273-313.
Sofowora. A, 1993, Medicinal plants and traditional medicine in Africa, 2nd Edition, Spectrum Books Ltd, Ibadan, Nigeria, 134-156.
Sørum, H., and Labée-Lund, T.M., 2002. Antibiotic resistance in food-related bacteria – a result of interfering with the global web of bacterial genetics. International Journal of Food Microbiology, Vol. 78, 43–56.
Srinivasa Rao, P. and K.S. Parekh. 1981. Antibacterial activity of Indian seaweed extracts. Botanica Marina 24: 577-582
Tanniou, A.; Vandanjon, L.; Incera, M.; Serrano Leon, E.; Husa, V.; Le Grand, J.; Nicolas, J.L.; Poupart, N.; Kervarec, N.; Engelen, A.; et al. Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J. Appl. Phycol. 2014, 26, 1215–1230.
Thanigaivel, S.; Hindu Vidhya, S.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Differential solvent extraction of two seaweeds and their efficacy in controlling Aeromonas salmonicida infection in Oreochromis mossambicus a novel therapeutic approach. Aquaculture 2015
Thanigaivel, S.; Vijayakumar, S.; Gopinath, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. In vivo and in vitro antimicrobial activity of Azadirachta indica (Lin) against Citrobacter freundii isolated from naturally infected Tilapia (Oreochromis mossambicus). Aquaculture 2015
Thanigaivel, S.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Antioxidant and antibacterial activity of Chaetomorpha antennina against shrimp pathogen Vibrio parahaemolyticus. Aquaculture 2014,
Trease GE, Evans WC, 2002, Pharmacognosy. 15th Ed.London: Saunders Publishers. pp. 42-393.
Verschwele, A., 2005. Weed control with herbicides – opportunities and risks for the Ecological Farming. In: Abstract of the 8th Scientific Conference on Organic Farming, Kassel, Germany, 1–4 March 2005.
Viso, A.C., D. Pesando and C. Baby. 1987. Antibacterial and antifungal properties of some marine diatoms in culture. Botanica Marina 30: 41-45.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biology"
Biology is the scientific study of the natural processes of living organisms or life in all its forms. including origin, growth, reproduction, structure, and behaviour and encompasses numerous fields such as botany, zoology, mycology, and microbiology.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




