Methane Detection Sensors: A Review
Info: 10333 words (41 pages) Dissertation
Published: 9th Dec 2019
Tagged: ChemistryTechnology
Methane Sensors: A Review
KEYWORDS: Methane Sensor, Electrochemical, Optical, Catalytic combustion, Pyroelectric, Semiconducting oxide, Sensitivity, Selectivity, Range
Abstract: In this paper a review of current methane detection technologies have been presented. The different type of methane sensors such as electrochemical sensors, optical sensors, catalytic combustion sensors, pyroelectric sensors and semiconducting oxide sensors have been discussed. Their detection mechanism, properties such as sensitivity, selectivity, cost, detection range, fouling issues and current technology readiness have been reviewed in this paper. An overall comparative analysis of each sensor on the basis of various parameters has been provided in the conclusion section.
- Introduction
Methane is a significant climate change forcing greenhouse gas with a short‐term impact 25 times greater than carbon dioxide.1 According to data from Canada’s United Nations Framework Convention on Climate Change (UNFCCC), oil and gas methane accounts for approximately 6% of Canada’s total GHG emissions when the 100 year global warming potential of methane is considered.2
Methane is the primary component of natural gas. As a result, methane emissions occur throughout the oil and gas industry, and these emissions are the largest anthropogenic source in Canada. Other than the effect on the environment, these emissions also account for a huge loss in revenue for the oil and gas industries. Up to 60% of methane loss is due to fugitive release with a significant percentage from leaky transmission lines (pipelines). In addition to natural gas exploration and transmission, methane is released and extracted during oil sands mining and processing activities. It is estimated that these activities release about 10% of the greenhouse gases (GHGs) generated in the extraction sector.3 In addition to being a potent GHG, methane build-up in enclosed spaces also poses a significant safety concern.
Although Canada only accounts for approximately 1.6% of the world’s GHG emissions, it is among the top 10 countries for absolute emissions. Standing firmly in the top three for estimated emissions per capita, Canada comprises 0.5% of the world’s population, but emits three times its population share.4
The objective of this review is to provide an overview of current methane detection technologies. The goal is to review various sensor technologies that are currently being used or can be implemented in the future to detect fugitive releases. Fugitive releases can be defined as leaks happening from highly pressurized equipments such as pipelines, valves, flanges of various unintended gases that might be harmful in general to the surrounding atmosphere and or economically as well.
It is projected that a 45% reduction in methane emissions can be achieved by monitoring unintended emissions through implementation of currently prevailing technologies. As reported in the Pembina Institute’s report, “ A 45% reduction of oil and gas methane is equal to 27 million tonnes of CO2 (56 Bcf of methane), and is achievable with existing technologies and techniques. This reduction comes at a net cost of $2.76 CAD per ton CO2 reduced.2 If the natural gas is valued at $5.00 CAD per Mcf. The methane reduced results in recovery of gas worth approximately $251.10 million CAD ($200.80 million USD) per year.”1
Canada played an active role at the Paris COP21 (21st Conference of the Parties) which led to a global agreement on mitigation, adaptation and financing of climate change action.4 The current government policy indicates that Canada will seek to fulfill its global responsibility in reducing GHGs .
In May of 2015, the government submitted its Intended Nationally Determined Contribution (INDC) to the UNFCCC indicating an economy-wide target of reducing GHG emissions by 30 % below 2005 levels by 2030.6
Reduction in current methane emissions will be a major step towards meeting the GHG reduction goals of the country. Methane sensor technology will play a vital role in this direction as it helps to detect fugitive emissions which account for a major part of the current emissions. The benefit of this technology is to detect the emission of GHG’s. “In Alberta, the oil and gas industry is the largest source of methane emissions. Methane emissions in 2014 from Alberta’s oil and gas sector were 31.4 mega tonnes of carbon dioxide equivalents. This accounted for 70% of provincial methane emissions and 25% of all emissions from the upstream oil and gas sector”5 . Methane sensor technology will also help Canada to fulfill its pledge of reducing its emissions to below 30% of 2005 levels by 2030.6
There are also benefits of methane sensor technology in detecting and thus reducing emissions from an economic perspective; the industrial segments of ownership such as oil and gas production can grow in terms of monetary sense by making money by reducing gas losses and also improve safety. This is also true in some other segments. Most midstream companies (gathering, processing, and storage) are paid a fixed fee for gas lost and consumed during their operations; a reduction in loss would result in a direct monetary profit. This paper is to review the current technologies with respect to methane detection with the objective of classifying current technologies by application, and highlight opportunities for future development.
- Sensor classification by range
This review paper focuses on methane sensors and tries to analyze various classes of sensors according to the following aspects
- Sensitivity: The ability to detect methane emissions at different concentration level and in different conditions.
- Range: The proximity level of the sensor at which it can detect the emissions.
- Technology Readiness: The level of commercial availability of the sensor, from lab scale to commercial purposes.
- Fouling: The resistance of the sensor towards contamination and the possible sources of contamination and reduction in efficiency
- Cost: The economic viability of the sensor. The future course of action to make the product more economic.
- Selectivity: The effect of the presence of other gases and the effectively of the sensor in detecting methane in presence of a mixture of gases and contaminants.
The paper discusses a variety of methane sensors based on different working principles. The sensors that will be analyzed throughout the paper are:
- Electrochemical Sensors
- Optical Sensors
- Catalytic Combustion Sensors
- Pyroelectric sensors
- Semiconducting oxide sensors
Methane sensors have a wide scope of application. They can be classified according to their use in specific fields. The sensors being developed on various working principals can be utilized for detection in specific areas.
- Electrochemical Sensors
- Ionic Liquid Electrochemical Sensors
Electrochemical gas sensors generally operate upon the principles of either differential electric double layer capacitance (DEDLC), or amperometry. A typical sensor design is modelled after the conventional three electrode system: a working electrode where the substrate will undergo redox, a counter electrode which balances the current at the working electrode, and a reference electrode with which to measure the working electrode potential against. In practice, most sensor designs compartmentalize the reference electrode to its own cell; however, the working and counter electrodes must be connected by an electrolytic fluid to maintain charge neutrality in the system. Aqueous electrolytes (AEs) are routinely used to fulfill this role since they are widely available, relatively cheap, and simple to use. Some examples of common AEs are sulfuric acid (H2SO4) and sodium hypochlorite (NaClO).
Despite their widespread use, aqueous electrolyte-based sensors (AESs) introduce a variety of problems into gas sensor design. First and foremost, AESs suffer from limited working lifespans primarily due to the volatility of the AEs used; evaporation of the AE requires that sensors of this design must be replaced more frequently than would be desired, translating to increased maintenance costs for the projects that employ them (mining, natural gas transportation, etc.). Of additional concern is the potential for interference through the electrolysis of water; in the practice of voltammetry, the potential range in which water is not electrolyzed is referred to as the “water window”, and overpotentials outside of this range will cause considerable loss of accuracy in the sensor. AESs also often require extreme process conditions to carry out the redox reactions required to sense certain analyte gases; methane oxidation can require process temperatures ranging from 775 °C down to 50 °C, depending on the type of electrolytes and catalysts used (i.e. yttria-stabilized zirconia7, lanthanide ruthenium oxide8, Nafion-sulfuric acid9, etc.). Additionally, methane oxidation is kinetically slow; this in turn has led to the design of systems utilizing hot acid electrolytes and platinum (Pt) catalysts, which give a reasonable oxidation rate. However, aside from the safety hazards associated with such a system, these designs also suffer from side-reactions which produce CO byproducts, poisoning the catalyst and reducing the lifespan of the sensor10. Another issue is that AESs generally suffer from significant signal interference from other common gases such as H2, NO, and C2H6.11 It is clear that while AEs are convenient and cheap to use, their associated drawbacks prevent AESs from reaching widespread commercial viability.
Ionic Liquids (ILs) are a class of electrolytes that have recently gained traction for use in gas sensor design; their unique properties bridge the gaps left by AESs, which affords new design pathways and functionalities. In particular, room temperature ILs (RTILs) have found increasingly common use in modern gas sensor designs since they circumvent the extreme process conditions required for certain oxidation pathways of methane analyte.12-13 Moreover, RTILs have higher boiling points than their AE counterparts; this inherent property overcomes the difficulty of dealing with solvent evaporation as is the case in AESs, making them an ideal electrolyte for sensor designs aiming to increase working longevity. Unlike many solid state electrolyte systems, RTILs do not suffer from electrical contact loss, and do not require significant pre-processing to integrate into the sensor design. Lastly, RTILs are less hazardous than the common acid electrolytes used in AES systems, making them ideal candidates for widespread production and use in industry.
1 – butyl – 1 – methylpyrrolidinium bis (trifluoromethylsulfonyl) imide, more commonly known as [C4mpy][NTf2], is one of the more frequently used RTILs in current methane sensor designs. Relative to other RTILs, [C4mpy][NTf2] features lower viscosity and higher chemical stability13; RTILs are already known to suffer from low gas diffusion rates due to their inherently higher viscosity when compared to AEs which, when compounded with the low kinetic favorability of methane oxidation, result in extremely slow methane oxidation rates. This makes [C4mpy][NTf2] more tolerable than other RTILs as an electrolyte. Additionally, as [C4mpy][NTf2] is hydrophobic, it reduces the potential for noise pollution from overpotential electrolysis outside of the water window.
One convenient feature of [C4mpy][NTf2] is that it changes the oxidative pathway of methane.12 Wang et al. showed in their experimental design that by using [C4mpy][NTf2] interfaced with a Pt electrode, they were able to cross-validate their methane sensing platform in situ via an oxygen reduction reaction; since the oxidation of methane and reduction of oxygen occur at two distinct potentials, this system of reactions provides a validation platform unique to the [C4mpy][NTf2]-Pt system that is not available in an AE-Pt system. In their paper, they propose the following redox pathway for their sensor design:
CH4+2O2→CO2+2H2O
(1)
2CH4+3O2→2CO+3H2O
(2)
O2+e→O2-.
(3)
2CO2+2O2-.→C2O62-+O2
(4)
The side reaction (2) produces CO that would eventually poison the Pt catalyst, as is the case in most AES systems. However, due to the presence of superoxide from (3), CO can be further reduced to CO2, and then finally to C2O62- via (4). It is shown in their results that the final product, C2O62- has insignificant interference with the methane signal and removes CO2 and CO from the system cleanly. Speculation holds that it is the IL’s ability to stabilize the superoxide which gives rise to this unique reaction pathway, as it is not found in any AES systems. Indeed, it can be seen that RTIL-Pt systems hold many advantages over the traditional AE-Pt systems.
- Solid Electrolyte Sensors
A variety of polymer electrolytes, metal oxides and inorganic salts have shown significant ionic conductivities over a good temperature range. Ionic conductors can be defined as solid electrolytes (SEs), when their electronic conductivities can be neglected. “The main principle applicable in solid electrolyte sensors is the establishment of the electrochemical equilibrium at the electrodes which results in a quantifiable equilibrium cell voltage (emf), according to the Nernst equation”14 . Sensors with an appropriate design for measuring can be developed by variation in properties like temperature and partial pressure.15
Narayanan et al developed a phosphate based proton conducting solid electrolyte hydrocarbon sensor. The use of sodium phosphate solid electrolyte along with a hydrogenation catalyst help detect methane at 600 C. The sensor was developed using a two compartment cell with gold electrodes painted with Pt/CeO2 and H2 gas where the solid electrolyte behaves as a proton conducting material exhibiting Nernst behavior.16
Zhu et al have utilized alkali orthophosphates as solid electrolytes for methane sensing at higher temperatures. One such solid electrolyte is sodium orthophosphate which according to Hooper et al acts as a versatile host for solid solution formation. This solid electrolyte has the capability of stabilizing at room temperatures and thus can sense hydrocarbon gases like methane at room conditions. Alberti et al have also successfully developed a methane gas sensor using proton conducting zirconium phosphates. This solid electrolyte also shows promising results of precise and sensitive detection.17-19
Potentiometric sensors, which are dependent on solid ionic conductors are able to demonstrate a variety of advantages that can be characterized by the measurement of their sensitivity, selectivity, and detection range. Galvanic cells, which consists of a two electronically conducting electrodes and an electrolyte (solid), reversible against both mobile and immobile ions of the electrolyte, are utilized for potentiometric measurements. 14
Amperometric sensors, in contrast to potentiometric ones, have a direct relationship of the electric signal with the gaseous concentration, temperature sensitivity (small), and the absence of any reference electrode. Due to their detection range, sensitivity and high temperature operation, amperometric sensors are widely used in controlling the operation of “lean-burn engines”.15Being solid state it is easy to perform at high temperatures. A variety of devices working at elevated temperatures have been developed involving the use of solid electrolyte sensor. Some examples being Sodium – Sulphur battery, the fuel cell (H2 – O2), based on yttria-stabilized zirconia (YSZ, O2 – conductor); and the oxygen concentration sensor, based on YSZ, to name a few.15 The SE methane sensors have gained interest in oil and gas industry where even minute leakage has a risk potential. These sensors are highly sensitive and have low response time thus making it suitable for such situations.
- Optical Sensors
Optical sensors use the absorption spectroscopy to detect certain chemical compounds such as methane gas.20 Infrared absorption spectroscopy is the most commonly used form of spectroscopy for optical methane sensors because it can be used to qualitatively analyze molecules by measuring the wavelength and intensity of the absorption of mid-infrared light.21 Certain chemical bonds have specific absorption band wavelengths making it possible to identify the composition of a given sample, for example the atmospheric transmission of methane has a wavelength of 2.3µm and 3.26 µm.22
A typical optical methane gas sensor is composed of a light source to provide the mid-infrared light, a tube to hold the gas sample being tested, an optical spectrum detector and a spectral filter. During operation mid-infrared light is emitted from the light source and reflects along the inside walls of the tube as it passes through the sample gas. As the light passes through the sample gas the gas molecules that vibrate within the infrared frequency range would absorb the infrared radiation provided from the light source. After passing through the gas the infrared light would reach the optical spectrum detector which would produce a measurement of the output intensity of the emitted light. Using both the initial intensity of the light source and the length of the sample which are known quantities along with the output intensity a value for the effective absorption coefficient of the gas can be determined. The effective absorption coefficient would be used to tell whether methane was present in the sample gas by comparing measured and literature values. The use of a spectral filter improves the measured value of the effective absorption coefficient because it blocks spectral regions that are not greatly affected by the sample gas causing the detector to focus on the regions of high absorption.23
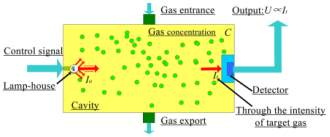
Figure 1: Gas Detection by IR Absorption Spectroscopy 29
The technology applied in the operation of optical methane sensors is already at the point where it is viable for industry. Currently there are multiple optical methane sensors on the market that are based on infrared laser spectroscopy. The optical sensors available on the market are belonging to one of two categories, either they are in-situ (on-site) sensors or they have remote sensing capabilities. The more common of the two are the in-situ sensors which require physical sampling of the air to be tested for methane detection. An example of an in-situ optical methane sensor on the market would be the mobile methane leak detector manufactured by Health Consultants Incorporated. This methane sensor was developed to detect methane along natural gas distribution, transmission and gathering pipelines and is mounted to a vehicle during operation. If methane is detected through infrared spectroscopy as the vehicle drives along the pipeline both an audio and visual signal is sent to a display inside the vehicle altering the operator. Similarly, Lechtzer Incorporated manufactured an optical methane sensor that is also attached to a vehicle and detects methane as one drives. There are also hand-held detectors that are used detection of methane leaks along pipelines. The remote sensing methane detectors still detect methane by infrared spectroscopy but don’t require physical sampling of a gas; instead the detector surveys a specific area and relays a signal through an optical fibre network when methane is detected. Applied Optoelectronics is an example of a company that manufactures remote sensing optical methane detectors for various industries.24
Even though there are many operational optical methane sensors on the market there are still lab scale experiments that continuously try to optimize the design. Typically the focus of these experiments is to improve the sensors selectivity, sensitivity and response time as these three fields are vital for sensing operations. Selectivity is important because there are many hydrocarbons at a similar wavelength as methane so the more sensitive the sensor is the more accurate it will be at detecting methane. Sensitivity is important so that the sensor will be able to detect methane concentrations at low levels and response time is important because in industry quickly detecting methane leaks is critical.
An innovation that focuses on increasing the sensitivity of the sensors is the use of a long period fibre grating (LPFG) optical sensor with a polycarbonate/ cryptophane A overlay deposition. LPFGs are used to couple light from a guided node towards forwards propagating cladding modes and can have their sensitivity increased through the use of overlays.25 In one experiment a polycarbonate/ cryptophane A overlay was used as an overlay due to its high refractive index. Altering the overlay thickness caused the resonant wavelength to shift and at optimal thickness there was found to be a drastic shift in resonant wavelength that resulted in a great increase in the sensor’s sensitivity.26 Through the use of the polycarbonate/ cryptophane A overlay the optical sensor was able to achieve a minimum methane detection limit of 0.2% on a volume be volume basis.27
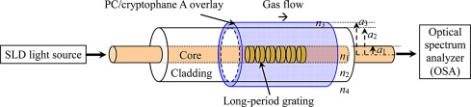
Figure 2: Basic Design of a LPFG Optical Sensor 26
Another innovation that is in the experimental phase is cavity enhanced optical sensors. According to theory, cavity resonance can be used to enhance the optical path length for absorbance spectroscopy applications.28 The experiment specifically uses a short confocal Fabry-Perot cavity in conjunction with moderately high reflectivity mirrors that provide many benefits such as easy injection of the laser bear into the cavity compared to other sensors that use cavities and decreases optical feedback from the cavity to the source laser.28 These benefits cause an increase in the absorption coefficient which leads the optical sensor to be highly sensitive as well as have a large dynamic range.28 Also it was found that by increasing the reflectivity of the mirrors the sensitivity of the sensor could be further increased allowing for a lower minimum detectable absorbance of methane. 28
Methane being dangerous towards human safety as well as the environment it is important to be able to detect it before problems arise. That is why the majority of the applications that optical methane sensors have in industry are to detect methane leaks from pipelines or to monitor levels in a given area.
Typically portable optical methane sensors are used for the detection of leaks in various industries such as oil and gas, water treatment plants and landfill sites because they have many advantages that allow for them to be mobile and be taken to the potential source of the leak.29 As mentioned previously there are many portable optical methane sensors that are on the market already that are attached to a vehicle and are used to detect methane leaks from natural gas pipelines.
Continuous optical methane detectors are used in applications where the methane levels in a given area are to be monitored. An example of this is the Remote Fibre Optic Methane Detection System designed by RSL Fibre Systems for the monitoring of coals mines. Since it can be dangerous for manual detection of methane emissions a remote fibre optical methane sensor is used to monitor the methane levels. Another example of a continuous optical methane detector is used for the monitoring of environmental methane emissions from a liquefied natural gas terminal in Dunkirk, France.29 Continuous monitoring of pipelines for methane leaks is a very large and important environmental application because it can lead to a reduction of greenhouse gas emissions.
The detection of methane gas leaks is very important for many industries and results in a large demand for precise but low cost sensors. Optical methane sensors are good for these industrial applications because of their low cost when compared to other methane sensors due to the fact that the main working principle behind optical methane sensors is absorption spectroscopy. Absorption spectroscopy requires much lower costs than other forms of analysis such as mass spectroscopy or gas spectroscopy.30
Optical methane gas sensors and calorimetric gas sensors have similar low production costs but where calorimetric sensors are non-selective, optical methane sensors have the advantages of being highly selective like gas spectroscopy based methane sensors which have high costs.31 One of the main reasons that optical methane gas sensors have low costs is because they have a low cost of ownership or in other words optical sensors are non-destructive.28 Since absorption spectroscopy is a physical process not a chemical reaction during operation the actual sensing apparatus is not damaged and does not require replacing unlike other methane sensors that use chemical reactions to detect the methane gas. The bulk of the cost associated with optical methane sensors is the manufacturing cost itself. The cost of production would increase if there was a need to increase the sensitivity of the methane sensor as more individual components would be needed, for example having two detectors in the sensor instead of one. Also the cost of production would also increase if there was a need to increase the selectivity of the sensor by adding a spectral filter or narrowing the bandwidth and tolerance of a spectral filter already in use.28
Optical methane sensors are useful for many industrial applications because they provide many advantages with few disadvantages when compared to other methane sensing technologies. One of the biggest advantages of optical methane sensors is that absorption spectroscopy is a physical analysis method not a chemical reaction which makes optical sensing a non-destructive method of detecting methane. This means that the components do not get degraded after repeated operation which in turn reduces any maintenance costs that other methane sensors would have to deal with. Another important advantage that optical methane sensors have is that they are immune to any electromagnetic interference which in other sensing techniques would impair the sensor from operating properly.26 Also optical methane sensors are very versatile and can be adapted to fit the requirements of various applications. There are portable designs available allowing for use in different environments and whether the application is detecting a possible leak or monitoring a specific area the sensors can be designed to be manual or remote sensing. Some other advantages include the ability to operate in the absence of oxygen, have fast response time, does not require any pre-treatment or build-up of gas sample, low cost and have good selectivity and sensitivity. The table below lists the sensitivities of various optical sensors currently available.
| Method | IR Source Wavelength | Sensitivity |
| Fabry-Perot Cavity27 | 1650 nm | 0.7-2.9 ppm-m |
| Interband Cascade Laser30 | 3291 nm | 1.0-2.1 ppm-v |
| QEPAS22 | 2300 nm | ~ 15 ppm-v |
| Infrared LED28 | 1660 nm | ~ 100 ppm |
| LPFG26 | 1550 nm | ~100 ppm |
The main disadvantage of optical sensors is that to be effective the target gas should have a significant and distinct optical absorption region. The problem is that methane has absorption bands in the same spectral region as many other hydrocarbons.28 This issue can be easily overcome by increasing the selectivity of the sensor by adding a spectral filter, however this would lead to an increase in the production costs of the sensor.
- Catalytic Combustion Sensors
Catalytic bead sensors have been in use for almost a decade and are primarily used to detect combustible gases, more generally in industrial safety instruments. They were initially used for the detection of methane and other gases in coal mines.32 Combustible gas mixtures will not burn until a certain ignition temperature but in the presence of a particular chemical process, the gas will burn or ignite at lower temperatures. This process is known as a catalytic combustion. Many metal oxides and compounds have these catalytic properties to help the combustion process such as platinum and palladium.
The basic principle is that combustible gases can be oxidized to produce heat. To apply the catalytic combustion process, a catalytic element such as a platinum coil is embedded in a catalyst. A catalytic palletized resistor (also known as a “pellistor”) consists of a platinum wire embedded within a ceramic pellet. On the surface of the pellet is a layer of high surface area noble metal, which when hot, acts as a catalyst to promote exothermic oxidation of combustible gases. Pellistors are manufactured in pairs where one acts as the detector while the other acts as a compensator for a reference resistance to remove the effects of environmental factors33. When a combustible gas comes in contact with the catalyst surface, it is oxidized and the reaction releases heat34. This results in a temperature resistance change within the coil and causes the resistance of the coil to change. The electrical circuit used to measure the output of the catalytic sensor is called a Wheatstone bridge as seen in Figure 3.32 During the burning of the gas of interest, it causes an imbalance of the bridge circuit. The offset voltage is measured as the signal and can be used to measure resistance, capacitance, inductance, impedance, and concentration.
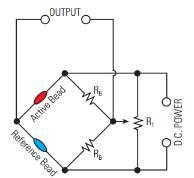 Figure 3: A catalytic bead sensor Wheatstone bridge.
Figure 3: A catalytic bead sensor Wheatstone bridge.
The catalytic gas sensor is a popular due to its simplistic design, easy to manufacture, and being inexpensive. Environmental conditions such as temperature, humidity, and pressure variation can influence both beads such that the Wheatstone bridge will not be unbalanced.33 This property to compensate for variations makes it possible for the catalytic gas sensor to operate in harsh environmental conditions.
The calorimetric gas sensor measures the heat of combustion during an oxidation reaction on the sensor surface. The exothermic nature of the combustion causes a rise in temperature. These devices are also known as pellistor, catalytic bead, catalytic gas sensor, combustible gas sensor, or thermometric gas sensor.35 Platinum, palladium, and rhodium are the most used catalysts in calorimetric gas sensors. Considering various fuel gas compositions, these sensors must be able to properly combust H2, CO, and CH4. The detection of CH4 is challenging as it requires a higher catalyst temperature.36
Gas calorimeters are used to control the thermal input in various types of steel mill and petrochemical industry furnaces, as well as in power plant turbines. Conventional calorimeters for fuel, nature gas, or coal gasification products are used for monitoring the heats of combustions of fuels in burners and turbines in industrial settings. Typical calorimeters are large, self-standing units that are used to monitor large-scale systems.36
In a study conducted by Akamastu et al., a new sensor type containing the functions of a conventional thermocouple-type calorimeter was developed. The device included a temperature sensor, catalytic combustor, and heater. It combined catalytic combustion and thermoelectric techniques – made suitable for low-cost production and advantageous in terms of short start-up and response time .36 The device was mainly used to detect CH4 due to its challenges.
New Cosmos – BIE offers a catalytic combustion sensor that utilizes a platinum coil.37 SRI Instruments Europe’s catalytic combustion detector consists of a coil of platinum wire embedded in a catalytic ceramic bead to detect hydrocarbons and H2 gas.38 Finally, Figaro Engineering Inc. offers various catalytic sensors to detect methane, propane, and iso-butane.39
The original catalytic sensor was a coil-shaped platinum wire to obtain a compact geometry for efficient heating. Unfortunately, at high temperatures, platinum start to evaporate. This causes a reduction in the cross-section area of the platinum and increases resistance. To improve the stability of the sensor is to coat the platinum wire with suitable metal oxides: treating the finished sensor or bead with a catalyst such as platinum, palladium, or theoria compounds. The reference wire should ideally be the same as the active wire, with the same geometry and operating temperature. It is not practically possible to do so but various compromises have been considered. Operating the reference wire at a substantially lower temperature and chemically treating the reference wire with gold are examples of ways to accomplish this. Using a fine diameter wire will reduce the size but increase the signal of the sensor while reducing the power consumption.32
In a study, micro-electromechanical systems (MEMS) technology was used to create smaller catalytic combustion gas sensors to decrease its power consumption significantly. The fabricated catalytic sensor possessed a relatively high response, selectivity, rapid response/recovery, and high reproducibility of the output signals. It also demonstrated its potential in portable sensing devices such as gas analyzers and gas leak detectors.40
One way to optimize a sensor for the detection of a large hydrocarbon molecule is to etch the bead more deeply and create larger channels for the gas to diffuse and reach the active sites. Since the heated molecules produced in the oxidation reaction escape more easily, there is less attenuation resulting in a higher relative response of the sensor.41 There are several calibration strategies to prevent incorrect readings due to the loss of sensitivity to methane. One method is to calibrate the sensor to a known concentration of methane. The relative response factor for methane can be used to determine if there is a loss of sensitivity.42 This can then be used to calibrate the instrument to make up for the loss in sensitivity.
The catalytic combustion sensor was initially used for the detection of methane and other gases in coal mines. Nowadays, applications include detection of combustible gases in industrial safety instruments, controlling thermal input in various furnaces in the steel mill and petrochemical industries, power plant turbines, and to monitor the heat of combustions of fuels in burners and turbines in the industrial setting
In detecting combustible gases, the two most common gas sensing technologies are catalytic combustion and infrared. Both consist of their own set of advantages and disadvantages. Comparatively, the catalytic detector has a low replacement cost and is much less expensive than the infrared. There is a wide variety of combustible gases and vapors that can be detected with the catalytic bead sensor but are limited to areas where sensor poisons are unlikely to occur. The cost-benefit ratio is very good if the purchase costs are taken into account but are less positive if frequent maintenance/replacement is required due to poisoning.43 The infrared technology has a higher spare parts cost but these types of sensors will be immune to contamination, poisoning and have a long lifespan while the catalytic combustion sensor can become poisoned due to contamination.44 While the infrared sensor can be used in locations where sensor poisons can occur, there are other combustible gases and vapors apart from hydrogen which cannot be detected with this technology.
The general cost can range from $1-$3 USD for individual sensor units up to $100-$1000 USD for prebuilt-incased sensors. Prebuilt-incased infrared gas sensors can range from $300-$2000 USD.
Catalytic combustion sensors have various advantages including a low cost, low replacement cost, ability to detect a wide variety of combustible gases and vapors, simplistic design and easy to manufacture. Additionally, it can be very portable and has a good selectivity for methane and other volatile hydrocarbon vapors.45 Compared to the infrared technology, it is much cheaper if operated in the correct setting as the lifespan can be drastically shorten if contaminated or poisoned.
The disadvantages include not being able to operate long-term, requires elevated temperatures, catalyst poisoning, sensor inhibitors, and cracking.44There are poisoning compounds which can deactivate the sensor by permanently reducing sensitivity until eventually completely damaging the sensor, resulting in the sensor becoming nonresponsive to gases.
- Pyroelectric Sensors
Pyroelectric sensors involves the conversion of thermal energy into electrical energy. To do this pyroelectric sensors employ the use of a dielectric substrate and a pyroelectric detector that is sandwiched between two electrodes. In pyroelectric sensors the dielectric substrate is an electrical insulator that when an electric field is applied becomes polarized and typically has a high thermal conductivity to minimize any heat loss.46 The detection process for pyroelectric sensors can be divided into a few main stages, the first stage being the generation of the thermal energy or wave that will propagate through the substrate which is typically caused by either a resistive heater or infrared light source.
The resistive heater generates the thermal energy by passing an electrical current through a highly resistive material, as the current flows it generates heat at a steady rate that can travel through the substrate. The thermal wave travels through the gas of interest until it reaches the pyroelectric detector located across from the resistive heater. When a pyroelectric material undergoes a temperature change it produces an electrical current that can be measured.47 This current has a corresponding voltage which can be used to find the composition of the gas of interest because both the voltage and phase of the output signal are a function of the gas’s thermal conductivity and diffusivity.48 The other method causes a temperature change in the pyroelectric material by focusing an intense infrared light source on a pyroelectric thin film.48Once the pyroelectric material changes temperature the sensor operates in the same way as the electric heater method mentioned previously.
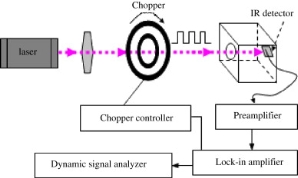
Figure 4: Basic Design of Pyroelectric Sensor based on Infrared Heating48
The technology used in the operation of pyroelectric methane sensors is already past the experimental phase of development and can be found on the market. There are multiple pyroelectric sensors used to detect gases including methane available that are based on the pyroelectric effect. An example of this is the pyroelectric detector developed by InfraTec that uses single-crystalline lithium tantalate as its think film pyroelectric material. This pyroelectric detector is very versatile as it can be used for methane detection as well as many other applications such as motion detection and flame detection. Another example is one of the smallest pyroelectric sensors on the market manufactured by Pyreos Sensing solutions called the ezPyro. This pyroelectric sensor uses lead zirconate titanate as its pyroelectric material and provides high sensitivity, fast response times and low power consumption. The ezPyro is versatile just like the sensor by InfraTec and can be used for flame detection, motion detection, gesture detection as well as gas sensing. For gas sensing applications there are specific filters that can be used to target the detection of specific gases such as methane.
Even with pyroelectric methane sensors already on the market there are still many experiments being run to improve the design by increasing the overall sensitivity and reducing the sensor’s power consumption. Sensitivity is always important quality for gas sensors to have because the target gases are typically in very low concentrations so the sensor must be able to pick up these minuscule readings. Also low power consumption is an important factor since many applications require the pyroelectric sensors to be portable, for example they might need to be taken to the location of a possible methane leak from a pipeline.
One innovation aims to increase the sensitivity of pyroelectric sensors through the concept of internal thermal amplification. Due to many beneficial characteristics such as long term stability and reproducibility this sensor uses lithium tantalate as its pyroelectric material but unlike typical pyroelectric sensors that would form the material as a simple thin film this design takes a different approach to improve sensitivity.49 In this design a matrix of holes are etched into the lithium tantalate resulting in thick electrically active areas and thin electrically passive areas. This is where the concept of internal thermal amplification comes into effect, when the pyroelectric material is hit by a thermal wave it creates a temperature gradient between the thin and thick areas causing the heat to flow from the passive to active areas.49 According to the pyroelectric effect that states when a pyroelectric material undergoes a temperature change it produces a current, therefore the thicker electrically active area of the lithium tantalate would undergo a larger temperature change and produce an increased output signal compared to that of a simple thin film sensor. From this study it was found that altering the structure of the pyroelectric material in this way could increase the sensitivity/responsivity of the sensor by 150%. 50
Due to their characteristic pyroelectric sensors are applicable for a variety of industries. Since they provide high responsivity and excellent detection pyroelectric sensors are used in applications such as fire alarms, laser detectors, thermal analyzers and gas analysis.51
Pyroelectric sensors are used for environmental applications such as gas analysis and pollution control because there is a need to quickly detect leaks and accurately determine the composition of the leak itself. This makes them a good choice for the oil and gas industry, water treatment plants and landfill sites where the detection of methane is a large safety and environmental concern.52Also another reason why pyroelectric sensors are used for these applications is that they can be designed to be a portable or remote-sensing sensor depending on the situation. Typically pyroelectric sensors that are based on infrared heating are used for applications that require remote sensing such as gas monitoring in coal mines. Detection of gases in coal mines can be very dangerous which is why it is beneficial if monitoring can be done from a remote location and infrared pyroelectric sensors make that possible.50 Portable pyroelectric sensors are useful for applications where there is a need to check for possible leaks and can be based on electric heater or infrared principles.
As previously stated there are many applications that pyroelectric sensors can be used for but to have any practical use there are certain criteria that have to be met. For pyroelectric sensors to be an effective method of methane detection in industry they must be low cost to allow for easy availability and widespread use.
The main cost benefit of pyroelectric methane sensors as opposed to some other sensors such as photon sensors is that they do not require any cooling.52 Pyroelectric methane sensors that use infrared light detection methods work well at room temperature unlike other sensors types that require a form of constant cooling. This eliminates the need to have any additional equipment to cool the sensor which in turn greatly reduces operational costs.51
The area in which pyroelectric sensors suffer from regarding cost is power consumption. Most pyroelectric sensors consume quite a bit of power to function which leads to increased operating costs. The cause of the large power consumption is due to the fact that to operate pyroelectric sensors require a constant source of the thermal energy for gas detection. If an electric heater is used for this a constant current must be applied which increases the power consumption or even if infrared detection is used a constant light source is required. This heavy power consumption is one of the challenges in designing a feasible portable pyroelectric methane sensor and is a high priority research area.52 Also unlike optical sensors, pyroelectric sensors operate based thermal process so there can be degradation of components over the sensors lifetime which would require either replacing parts or the sensor itself further increasing cost.
Pyroelectric methane sensors can be very useful in industrial applications because they provide many advantages that distinguish them from other sensor types. One main advantage is that pyroelectric sensors are able to operate at room temperature.52As mentioned previously this reduces operating costs by eliminating the need for cooling equipment but it also enables pyroelectric sensors to be used in a variety of environments and climates. To add to its versatility pyroelectric sensors can function in environments absent of oxygen as well as inert atmospheres. Another advantage of pyroelectric sensors is that the technology they are based on is a physical process. The pyroelectric effect is a thermal process and does not involve any chemical reactions like some other sensor types which could lead to degradation. Some other advantages that pyroelectric methane sensors have are high sensitivity, easy miniaturization, wide measuring range and excellent responsivity. The table lists the sensitivities of the sensor currently available.
| Method | Material | Sensitivity |
| NDIR53 | Mgg lamp (6004-10) | ~ 1 ppm |
| Thermal Wave Pyroelectric Film47 | organic polycyclic molecular crystalline substance | ~ 50 ppm |
Pyroelectric sensors also have some disadvantages, the main one being the high power consumption compared to other sensors. As stated previously the power consumption is a result of a need for a constant current or constant infrared light source to provide the thermal energy required for methane detection.52Some other minor disadvantages would be slow degradation of components due to repeated heating and the potentially high operating costs.
- Semiconducting Oxide Sensors
Semiconducting metal oxides (SMO) are electrical conductivity sensors that detect gas by a REDOX reaction when a gas of interest comes in contact with the sensor. The gas of interest will absorb onto the sensor’s active sensing layer to cause a change in the resistance; the concentration of the gas can be determined from the resistance change.54 They are relatively inexpensive compared to other sensing technologies, robust, lightweight, long lasting, and benefit from high material sensitivity and quick response time.55 Due to their chemical compositions and properties, SMOs are well-suited for a wide range of applications and for all reactive gases.
A SMO sensor possesses an electrical resistance made with a porous assembly of tiny crystals of n-type metal oxide semiconductors, such as SnO2 or WO3.56 When the sensor is heated to a high temperature in the absence of oxygen, electrons will flow through the grain boundaries of the SMO film. When oxygen is present, it will absorb onto the SMO surface to form a potential barrier. This interaction forms ionic species including O2–, O– and O2-, which trap electrons from the bulk of the material. It also repels other electrons from the bulk of the film, creating a region with a reduced amount of electrons, thus resulting in an increased potential barrier at the grain boundaries. This impedes the flow of electrons and increases the resistance. When the sensor is exposed to an atmosphere containing a reducing gas such as CH4 and H2S, the SMO surface absorbs the gas molecules and lowers the potential barrier, thus increasing the concentration of electrons at the surface while reducing the electrical resistance57. In the case of oxidizing gases such as NO2 and CO2, the electrical resistance will increase. Several researchers have reported that the conductivity response is highly affected by the presence of a catalyst, which will increase the surface reactivity.58 Figure 5 is a schematic of a SMO sensor design during a study conducted for the United States Environmental Protection Agency’s Environmental Monitoring Systems Laboratory.59
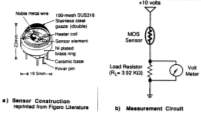
Figure 5: Schematic of a metal oxide semiconductor sensor.
In the case of SMO sensors for CH4 gas, a number of metal oxide materials have been studied as seen in Table 157:
Table 1: Gas sensing properties of unloaded/loaded metal oxide semiconductors for methane gas.
| Sensing Material | Method | Gas Concentration | Sensing Response |
| 7 wt% Sb/ZnO | Screen printing | 1000 ppm | ~25 to 1000 ppm at 360oC |
| SnO2 | Thin films on micromachined SOI wafers | 1%/room temperature – 700oC | ~23 at 500oC to 1%
Response time: 5-10 ms |
| SnO2-ZnO (0-15 wt% ZnO) | Thick films | 50 ppm/170-330oC | ~1 (10% ZnO loaded SnO2) at 300oC to 50 ppm
Response time: ~2s |
| Unloaded WO3 | RF-sputtering (sensors) | 1000 ppm | < 0.5 to 1000 ppm at 300-500oC |
| Unloaded SnO2, Pt/SnO2, Mo/SnO2, Cu/SnO2 | Thin films on alumina substrates by CVD | 1000 ppm/450-500oC | 0.4, 0.72, 1.03, 1.50 to 1000 ppm, at 500oC respectively
Response time: ~5s |
Conductometric semiconducting metal oxide sensors have been widely used in various domestic, commercial, and industrial gas sensing systems. They currently constitute one of the most investigated groups of gas sensors due to their low cost and flexibility in production, wide variety of detectable gases, and lightweight.60 In addition, SMO sensors tend to be long-lived and resistant to poisoning. For these reasons, they have grown rapidly in popularity to become the most widely used gas sensors available. They are used in environmental monitoring and gas detection, carbon monoxide sensors, and breath analyzers. Most common detected gases include nitrogen oxide gases (NOx), H2S, NH3 and amine sensors, hydrogen, ozone, carbon monoxide, and methane gas.
Various companies have developed and currently market their sensors. SGX Sensortech’s marketed SMO sensors detect various gases including nitrogen dioxide, carbon monoxide, hydro carbons, volatile organic compounds (VOCs), and ammonia.61 Figaro Engineering Inc., a company specialized in research, development and manufacturing of gas sensors, has developed various SMO sensors for domestic and industrial safety to detect methane, butane, and propane.62 BACtrack is a leader in breathalyzers that utilizes either fuel cell sensor technology or semiconductor oxide sensor technology to measure the blood alcohol content (BAC) in a person’s blood.63
Researchers have determined that the interaction of the gas with the surface of the material is a characteristic of semiconducting metal oxide gas sensors. The reaction can be influenced by many factors such as natural properties of base materials, surface areas and microstructure of sensing layers, surface catalyst, temperature and humidity, etc. The principle of the classical semiconductor gas sensors is well known since the development of the “Taguchi-type” sensors.64
In 1967, Shaver described effects achievable with oxide semiconductors modified by the addition of noble metal catalysts such as Pt, Pd, Ir, and Rh. At the start of 1970s, Taguchi fabricated and patented the first chemoresistive gas sensor device using tin dioxide (SnO2) as the sensitive material.65After investigating various metal oxides, SnO2 was determined to have many advantageous properties such as higher sensitivity, low operating temperature, and a thermal stable structure. Platinum (Pt) was also used as a metal catalyst In order to increase sensitivity, selectivity and stability. The main application of this device was used by Figaro Engineering Inc. as detection alarms by monitoring the presence of hazardous levels of explosive gases. Additional research on semiconductor metal oxides used as sensing materials include: SnO2, ZnO, TiO2, WO3, Fe2O3, CuO, NiO, Cr2O3, etc.65 ZnO has become popular due to its high mechanical and chemical stability, suitability to doping, non-toxicity and low cost. TiO2 is particularly attractive because of its lower cross-sensitivity to humidity compared to other metal oxides. The Siemens Corporate Technology focused on the development of new sensing materials such as Ga2O3, SrTiO3 thin films and WO3/TiO2 mixed oxides.64They are very chemically robust compared to the classic SnO2 sensing material and are operated at higher temperatures. This enables the usage of these semiconductor gas sensors in chemically harsh conditions like exhaust gas and industrial environments.
Doping the metal oxide layer with suitable promoters enhances the sensing characteristics of the oxide semiconductor. For example, doping SnO2 with Sb, Cl, and F increases the conductivity. Depositing SnO2 on titanium presents high overpotentials for the oxygen generation reaction, giving good anodes for the electrochemical oxidation.66To modify or control the surface properties of semiconductor metal oxides, the usage of noble metal additives can be introduced. The most important effects of the additives are to increase the maximum sensitivity and rate of response, as well as lowering the temperature of maximum sensitivity
Semiconductor gas sensors can be used for a wide variety of applications, ranging from safety equipment (explosion, leakage, fire, contamination, and poisoning protection), air quality monitoring, quality assurance, and measurement technology. Unfortunately, they are susceptible to contaminants and changes due to environmental conditions and non-linear response effects complexity. Additionally, solid state metal oxide semiconducting gas sensors can be susceptible to background gasses, or have cross-selectivity and is sensitive to changes in humidity. There are options and configurations to help mitigate the sensitivity but it is an inherent characteristic of the sensing technology.
- Conclusion:
| Sensor |   Electrochemical Electrochemical
Solid Electrolyte Ionic Liquid |
SMO | Catalytic Combustion | Optical | Pyroelectric | ||
| Criteria | Multiplicative Factor | ||||||
| High selectivity for methane | 3 | 9 | 7 | 3 | 2 | 10 | 4 |
| High sensitivity for methane | 2 | 9 | 4 | 7 | 7 | 7 | 5 |
| Fouling resistance (temp, corrosion, poison resistant) | 3 | 8 | 4 | 3 | 1 | 7 | 7 |
| Lifetime | 3 | 5 | 5 | 7 | 7 | 8 | 6 |
| Easily manufactured | 1 | 7 | 6 | 8 | 8 | 4 | 6 |
| Low cost | 3 | 7 | 10 | 10 | 7 | 1 | 7 |
| Good detection range | 2 | 8 | 6 | 7 | 10 | 5 | 2 |
| Technology readiness | 1 | 4 | 9 | 3 | 7 | 10 | 8 |
| Final Score | 132 | 113 | 108 | 100 | 116 | 100 | |
This review paper successfully discusses and analyzes the various types of methane sensors and their technologies. The paper carefully evaluates the various aspects of each and every category. The sensitivity, technology readiness, selectivity, cost and fouling aspects of the sensors are taken into consideration and a comparative analysis is done in each case. The analysis of various sensors have been summarized below in the table in terms of a graded version for easier understanding purposes.
After reviewing the various sensors, it can be concluded that the optical sensors have the highest availability and acceptability in the current market. The technology is ready for market and there are lesser issues with respect to fouling, sensitivity and selectivity for methane. The optical sensors are expensive and are not very effective for underground pipelines or difficult terrains. Issues like these can be overcome by Ionic liquid and semiconducting oxide type sensors as they are cheap, have good selectivity and good detection range as well. The research on these sensors is currently based on addressing issues like selectivity and fouling. In the near future, these sensors have the potential to dominate the market and lower down the detection costs as well.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Technology"
Technology can be described as the use of scientific and advanced knowledge to meet the requirements of humans. Technology is continuously developing, and is used in almost all aspects of life.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




