Behavioural Traits in Horse’s Equus Caballus to Novel Stimuli
Info: 10828 words (43 pages) Dissertation
Published: 16th Dec 2019
Tagged: Animal Sciences
Behavioural traits in horse’s Equus caballus to novel stimuli
Table of contents
Introduction……………………………………………………………………………………………………………………………………………………………
Methods & Materials…………………………………………………………………………………………………………………………………………………….
Results………………………………………………………………………………………………………………………………………………………………………….
Discussion……………………………………………………………………………………………………………………………………………………………………..
References…………………………………………………………………………………………………………………………………………………………………
Introduction
Importance of horses/ human interaction with horses
The horse Equus caballus has co-existed alongside humans with evidence of domestication taken place approximately 3000 B.C. to 2500 B.C. (Zeuner, 1963). The variations of breeds in the horse species is not completely the result of natural selection but is the creation of horse breeders. Humans have interfered with breeding of the horse due to the restriction of movement and breeding pressure has conflicted evolutionary process of behaviour (Goodwin, 1999). Despite human manipulation interfering with the natural process, the behaviours exhibited by these animals has resulted from the horse’s natural past (Clutton-Brock, 1999).
In present day the horse is used in sports and leisure, however the sport ranks as one of the most dangerous sports to be in. Fear reactions within horses can cause serious injuries in both humans and horse, the ability for the horse to habituate to a range of novel stimuli greatly increases safety in the horse-human relationship (Christensen, 2006).
It is often emphasised that equine stereotypies for example as crib-biting, wind-sucking and weaving are instigated by boredom (Nicol, 1999). Due to the horse training ensued the knowledge of the scientific evaluation of equine mentality, these are known via the traditional term of sterotypies- vices (McGreevey & Nicol, 1998). The behaviours known as vices of horse ethology, are treated and considered undesirable for economic and cultural reasons and or not treated due to these activities affecting the horse quality of life (Cooper & Mason, 1998).
Horses learn via trial and error, if horse handlers prevent the natural behaviour of a horse when faced with a novel stimulus can result in dangerous behvaiours; rearing, bucking and shying collective known as evasion (McGreevey & McLean, 2005).
Some of these behaviors are escape behaviors; others are forms of self-stimulation. Most can be eliminated by pasturing rather than stall confinment. Trailering problems include failure to load, scrambling in the moving trailer, struggling in the stationary trailer, and refusal to unload. Gradual habituation to entering the trailer, the presence of another horse, or a change in trailer type can be used to treat these problems.
Houpt, K. A. (1986). Stable vices and trailer problems. Veterinary Clinics of North America: Equine Practice, 2(3), 623-633.
Leiner & Fendt, (2011) found that behavioural and physiological responses are correlated. After habituation training (form of learning that desensitizes to a stimulus after repeated presentation), the fear response is significantly reduced for that stimulus, whereas the fear response is sustained for another stimulus.
Leiner, L., & Fendt, M. (2011). Behavioural fear and heart rate responses of horses after exposure to novel objects: effects of habituation. Applied Animal Behaviour Science, 131(3), 104-109.
Temperament
The most common way to judge a horse “personality” is through its agonistic behaviour which transmits through; facial expression, postures, vocalisation and locomotion activities and signifies to the observer where on the scale the horse lies between dominance and submissive (Evans, 2000)
In prey species, it is especially likely that responses to suddenness are stronger than responses to novelty per se, due to similarities with moving predators. In this experiment, we separate novelty from suddenness, focussing only on the effects of novelty in a known environment.
Can link to horses being prey animals -evolution- importance to understand for safety
Laterization
The right hemisphere appears to be the control of agonistic behaviour and responses to novelty and the left hemisphere is a measure of behaviour of aggression and reactivity (Austin & Rogers, 2012; Austin and Rogers, 2014)
What is lacking in the field
There is a lack of research on fear reactions with no published research on basic habituation processes in horses (Christensen, 2006). Furthermore, there is very little research on different sensory stimuli effect the animal behaviour and does one sensory effect horse behaviour differently.
What does my study do that no others do
The aim
The aim of my study is to
Methods
Methods
In the Methods section, give enough detail to allow a competent scientist to repeat the experiments, mentally or in fact. Describe the preparation method, equipment, and measurements, including SI units. Cite references for your methods. If the techniques are widely familiar, use only their names. If a method is modified, outline the modification or cite a reference, unless the modification is trivial. Give details of unusual experimental designs or statistical methods. The Methods section may be arranged chronologically, by a succession of techniques, or in any other logical manner, such as by experiment or location, and may include tables and figures.
Housing/ stabling
All horses are housed in stables (Dimensions 12m×12m) over-night. During the day time they spend in paddocks when horses aren’t working, when working; they work in various environments of indoor arena (Dimensions 41m×21m), outdoor arena (Dimensions 62m×22m) and go out on hacks. The individuals observed (Table 1) in this study are on a diet mainly consisting of straw and hay, with supplements required if needed.
Table 1. Details of horses observed in the study.

Setting
To detect if there is difference between behaviours in horses when there is no stimulus present to the introduction of novel stimuli. In this study three novel stimuli where investigated; Visual, auditory and olfactory. A behavioural study was undertaken, observing Equus caballus behaviours, by recording the duration and frequency of behaviours (Table 2) exhibited by an individual. In the months of December- February at Margaret Haes Riding centre, horses where studied for 4 hours every 2/3 days. horses where observed within their stables.
Test procedure
Ten individuals were observed, with an observation time period of 10 minutes per individual, precision of measurement was to the full second. Behaviour was recorded using a stopwatch and lapping the time when the behaviour changes in the 10-minute time frame.
For each individual horse a control (novel stimuli was not present) was taken before the introduction of the novel stimuli. To detect the difference of duration and frequency of behaviour, a control was not taken after the 10 minute with the stimulus present as habituation period to a novel object varies from 0 to 4 minutes (Forkman et al., 2007). We can then detect the effects on horse behaviour after the initial introduction of novel stimuli, within the timeframe of the 10 minutes.
Visual stimuli
Red cardboard (60cm × 60cm) placed vertically upwards on a hazard cone 5 metres in front of the individual’s stable door.
Auditory stimuli
Talk radio sourced from a radio placed on the ground 2m in front of the individual’s stable door.
Olfactory stimuli
Lavender oil placed on a cloth placed over the individual’s stable door.
Table 2. Ethogram of horse Equus caballus behaviour
| BEHAVIOUR | DEFINITION |
| Locomotion | |
| Stand | Standing relaxed with head and neck relaxed |
| Walk | Walking at any pace |
| Trot | A pace that is faster than walking speed, in which diagonal pair of legs are lifted alternately. |
| Canter | Pace faster than a trot, with a minimum of one foot on the ground |
| Rolling | Movement of turning from one side to the other horizontally |
| Vocalisation | |
| Snorting | Abrupt forcing of large amounts of air out the nose |
| Neigh/Whinny | A long, loud and high call produced by a horse |
| Nicker | A soft whinny sound |
| Blowing | creating an air current from the nose or mouth |
| Vices | |
| Kicking | Contact with on object with the hoof or fast motion of leg movement in situ |
| Rearing | Horse stands on its hind legs with forelegs off the ground |
| Pawing | To strike or scrape on object with hooves |
| Wood Chewing | The continuous biting of wood |
| Retreat | Avoid from unfamiliar object or situation-minimum of one head length away |
| Shoving | Pushing objects using the head/ nose in a fast motion |
| Head toss | The sudden movement to move the head back from its normal position |
| Investigation | |
| Approach | Approaching an object- minimum of one head length movement to an object or situation |
| Biting | Exploration using the mouth for an object |
| Sniffing | Exploration of odour by inhaling with both nostrils |
| Head turn | Movement of head to one side observing with one eye |
| Flehman response | The action of the horse curling its lip upwards exposing the individual’s teeth |
| Vigilance | Standing still, with elevated neck, intently orientated ears and neck |
| Tail posture | The fleshy area of the tail is horizontal |
| Shoving (novel) | The gentle pushing of the novel object to investigate using the head/ nose |
| Other | |
| Resting | Cease all movement with all muscles relaxed lying on the floor |
| Elimination | The discharge of urine and defecation from the body |
| Yawning | The involuntary movement of opening the mouth |
Statistics
All data was recorded and entered into statistical software SPSS then analysed, to identify the statistical relevance of the effects of behavioural traits with the introduction of novel stimuli, using the Mann-whittney non-parametric analysis with data represented as median (Q1, Q3) of duration and frequency of each behaviour. The significance of the results was considered p value ≤ 0.05 with respect to the control, with n value corresponding to the number of individuals tested within each of the three treatment groups.
Results
Duration of observed behaviours
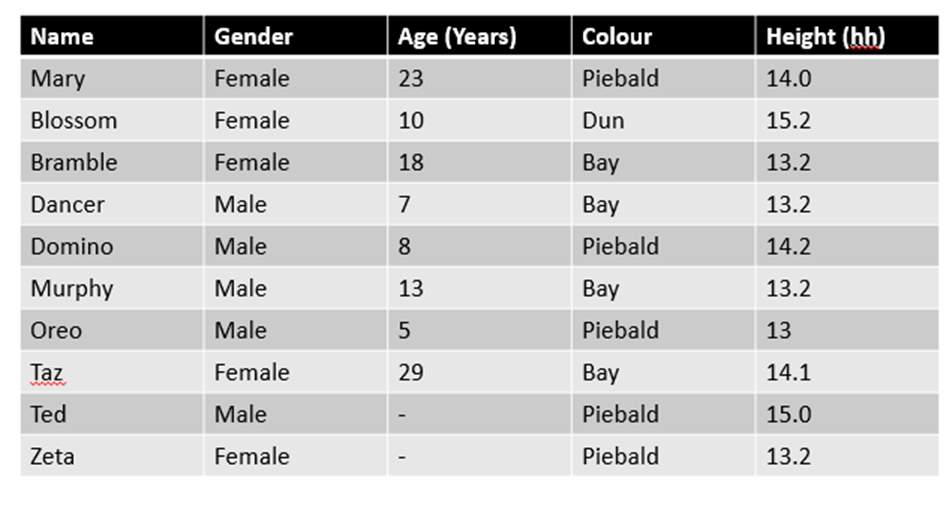
A. B.
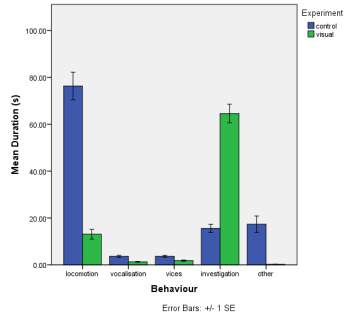
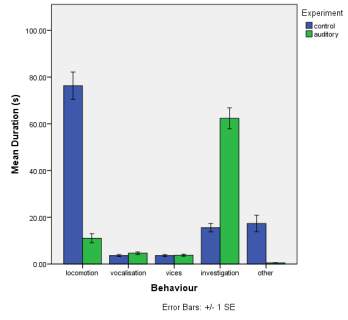
C.
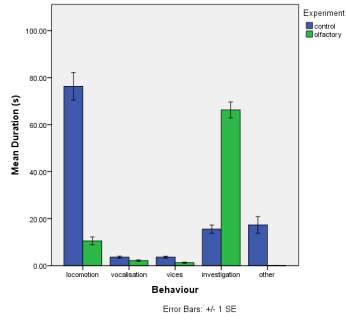
Figure 1. The mean (±SE) duration (s) of the observed behaviours; locomotion, vocalisation, vices, investigation and other of horse Equus caballus behaviours (n=10) for novel stimuli: visual (1A), auditory (1B) and olfactory (1C)
Table 3. The median (Q1, Q3) and statistical analysis of the duration (s) behaviours; Locomotion (Stand, Walk, Trot, Canter and rolling), Vocalisation (Snorting, Neigh, Nicker/Whinny and Blowing), Vices (Kicking, Rearing, Pawing, Wood-chewing, Retreat, shoving and Head toss), Investigation (Approach, Biting, Sniffing, Head turn, Flehmen response, Vigilance, Tail-posture and Shoving of the novel object) and Other (Resting, Elimination and Yawning).
| Behaviour | Control | Stimuli | |||
| Visual | Auditory | Olfactory | |||
| Stand | Median (Q1, Q3) | 243 (192, 400) | 0 (0, 100) | 0 (0, 98) | 0 (0, 100) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 1441.00 | 1286 | 1067 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Walk | Median (Q1, Q3) | 98 (0, 200) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 1972 | 1828 | 1903 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Trot | Median (Q1, Q3) | 0 (0, 0) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5610 | 5610 | 5610 | |
| P-value | – | 0.004 | 0.004 | 0.004 | |
| Canter | Median (Q1, Q3) | 0 (0, 0) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5610 | 5610 | 5610 | |
| P-value | – | 0.004 | 0.004 | 0.004 | |
| Rolling | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5830 | 5830 | 5830 | |
| P-value | – | 0.044 | 0.044 | 0.044 | |
| Snorting | Median (Q1, Q3) | 0 (0, 0) | 0 (0,0) | 0 (0, 0) | 0 (0, 4) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5655 | 6029 | 5038 | |
| P-value | – | 0.167 | 0.947 | 0.007 | |
| Neigh | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 23) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5579 | 4199 | 5824 | |
| P-value | – | 0.060 | <0.001 | 0.315 | |
| Nicker | Median (Q1, Q3) | 0 (0, 8) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4361 | 4726 | 4015 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Blowing | Median (Q1, Q3) | 0 (0, 4) | 0 (0, 1) | 0 (0, 5) | 0 (0, 3) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5342 | 5836 | 5797 | |
| P-value | – | 0.068 | 0.589 | 0.526 | |
| Kicking | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5722 | 4940 | 5885 | |
| P-value | – | 0.077 | <0.001 | 0.082 | |
| Rearing | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5885 | 5885 | 5885 | |
| P-value | – | 0.082 | 0.082 | 0.082 | |
| Pawing | Median (Q1, Q3) | 0 (0, 6) | 0 (0, 6) | 0 (0, 12) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5701 | 5516 | 4048 | |
| P-value | – | 0.398 | 0.190 | <0.001 | |
| Wood chewing | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5555 | 5555 | 5555 | |
| P-value | – | 0.002 | 0.002 | 0.002 | |
| Retreat | Median (Q1, Q3) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5830 | 4730 | 5445 | |
| P-value | – | 0.044 | <0.001 | 0.001 | |
| Shoving | Median (Q1, Q3) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5335 | 5909 | 5984 | |
| P-value | – | <0.001 | 0.611 | 0.796 | |
| Head toss | Median (Q1, Q3) | 0 (0, 26) | 0 (0, 0) | 0 (0, 4) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4626 | 5290 | 4653 | |
| P-value | – | <0.001 | 0.053 | <0.001 | |
| Approach | Median (Q1, Q3) | 0 (0,0) | 127(55,179) | 0 (0, 34) | 0 (0, 13) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 220 | 3850 | 4235 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Biting | Median (Q1, Q3) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4895 | 5170 | 5445 | |
| P-value | – | <0.001 | <0.001 | 0.001 | |
| Sniffing | Median (Q1, Q3) | 0 (0, 0) | 5 (0, 49) | 0 (0, 0) | 293(206,329) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4433 | 5030 | 154 | |
| P-value | – | <0.001 | 0.952 | <0.001 | |
| Head turn | Median (Q1, Q3) | 0 (0, 23) | 0 (0, 91) | 106(24, 308) | 50 (0, 61) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5016 | 1925 | 2855 | |
| P-value | – | 0.016 | <0.001 | <0.001 | |
| Flehmen response | Median (Q1, Q3) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) | 169(120,198) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5830 | 6050 | O | |
| P-value | – | 0.044 | 1.000 | <0.001 | |
| Vigilance | Median (Q1, Q3) | 90 (0, 222) | 307(266,401) | 386(285,439) | 11 (0, 107) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 1188 | 1280 | 4912 | |
| P-value | – | <0.001 | <0.001 | 0.011 | |
| Tail posture | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 1) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4780 | 5117 | 5555 | |
| P-value | – | <0.001 | 0.001 | 0.002 | |
| Shoving novel object | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0,0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5500 | 6050 | 5445 | |
| P-value | – | 0.001 | 1.000 | 0.001 | |
| Resting | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4950 | 5350 | 4950 | |
| P-value | – | <0.001 | 0.010 | <0.001 | |
| Elimination | Median (Q1, Q3) | 0 (0, 3) | 0 (0,0) | 0 (0,0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4940 | 5504 | 4400 | |
| P-value | – | 0.001 | 0.125 | <0.001 | |
| Yawning | Median (Q1, Q3) | 4 (0, 17) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 2302 | 2360 | 2200 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
Frequency of observed behaviours
A. B.
C.
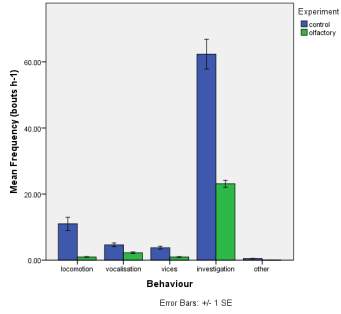
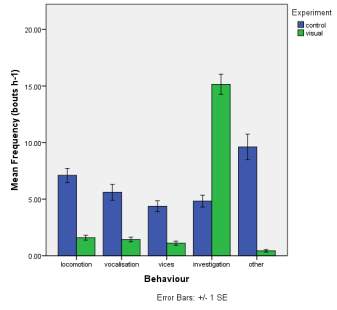
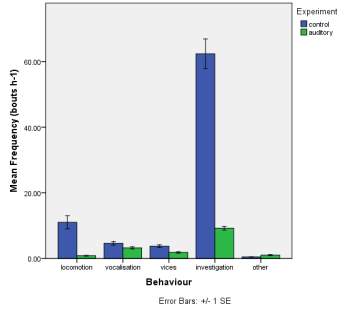
Figure 2. The mean (±SE) duration (s) of the observed behaviours; locomotion, vocalisation, vices, investigation and other of horse Equus caballus behaviours (n=10) for novel stimuli: visual (2A), auditory (2B) and olfactory (2C).
Table 3. The median (Q1, Q3) and statistical analysis of the duration (s) behaviours; Locomotion (Stand, Walk, Trot, Canter and rolling), Vocalisation (Snorting, Neigh, Nicker/Whinny and Blowing), Vices (Kicking, Rearing, Pawing, Wood-chewing, Retreat, shoving and Head toss), Investigation (Approach, Biting, Sniffing, Head turn, Flehmen response, Vigilance, Tail-posture and Shoving of the novel object) and Other (Resting, Elimination and Yawning).
| Behaviour | Control | Stimuli | |||
| Visual | Auditory | Olfactory | |||
| Stand | Median (Q1, Q3) | 12 (6,18) | 0 (0, 18) | 0 (0,6) | 0 (0, 6) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 3281 | 1920 | 2123 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Walk | Median (Q1, Q3) | 6 (0, 12) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 2012 | 1776 | 1865 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Trot | Median (Q1, Q3) | 0 (0, 0) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5610 | 5610 | 5610 | |
| P-value | – | 0.004 | 0.004 | 0.004 | |
| Canter | Median (Q1, Q3) | 0 (0, 0) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5610 | 5610 | 5610 | |
| P-value | – | 0.004 | 0.004 | 0.004 | |
| Rolling | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5830 | 5830 | 5830 | |
| P-value | – | 0.044 | 0.044 | 0.044 | |
| Snorting | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 6) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5571 | 6048.5 | 5066 | |
| P-value | – | 0.093 | 0.996 | 0.009 | |
| Neigh | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 6) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5777 | 4337 | 5847 | |
| P-value | – | 0.235 | <0.001 | 0.364 | |
| Nicker | Median (Q1, Q3) | 0 (0, 6) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4461 | 4839 | 4125 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Blowing | Median (Q1, Q3) | 0 (0, 12) | 0 (0, 0) | 0 (0, 6) | 0 (0, 6) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4862 | 5360 | 5594 | |
| P-value | – | 0.002 | 0.082 | 0.252 | |
| Kicking | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5610 | 5170 | 6050 | |
| P-value | – | 0.004 | <0.001 | 1.000 | |
| Rearing | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5885 | 5885 | 5885 | |
| P-value | – | 0.082 | 0.082 | 0.082 | |
| Pawing | Median (Q1, Q3) | 0 (0, 6) | 0 (0, 6) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5455 | 4992 | 4246 | |
| P-value | – | 0.140 | 0.006 | <0.001 | |
| Wood chewing | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5555 | 5555 | 5555 | |
| P-value | – | 0.002 | 0.002 | 0.002 | |
| Retreat | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5830 | 4730 | 5445 | |
| P-value | – | 0.044 | <0.001 | 0.001 | |
| Shoving | Median (Q1, Q3) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5500 | 5756 | 5995 | |
| P-value | – | 0.001 | 0.0266 | 0.819 | |
| Head toss | Median (Q1, Q3) | 0 (0, 42) | 0 (0, 0) | 0 (0, 6) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4343 | 4633 | 4225 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Approach | Median (Q1, Q3) | 0 (0,0) | 36 (12, 48) | 0 (0, 12) | 0 (0, 12) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 220 | 3850 | 4180 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Biting | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4895 | 5170 | 5390 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Sniffing | Median (Q1, Q3) | 0 (0,0) | 3 (0, 36) | 0 (0, 0) | 66 (54, 102) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4571 | 6030 | 990 | |
| P-value | – | <0.001 | 0.952 | <0.001 | |
| Head turn | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 36) | 21 (6, 48) | 18 (12, 24) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4377 | 2241 | 2400 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
| Flehmen response | Median (Q1, Q3) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) | 66 (54, 72) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5830 | 6050 | 0 | |
| P-value | – | 0.044 | 1.000 | 0.000 | |
| Vigilance | Median (Q1, Q3) | 6 (0, 36) | 30 (24, 48) | 36 (18, 36) | 6 (0, 60) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 3595 | 4081 | 5920 | |
| P-value | – | <0.001 | <0.001 | 0.771 | |
| Tail posture | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 6) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4895 | 5225 | 5555 | |
| P-value | – | <0.001 | 0.005 | 0.002 | |
| Shoving novel object | Median (Q1, Q3) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 5555 | 6050 | 5445 | |
| P-value | – | <0.002 | 1.000 | 0.001 | |
| Resting | Median (Q1, Q3) | 0 (0,0) | 0 (0, 0) | 0 (0, 0) | 0 (0,0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4950 | 5362 | 4950 | |
| P-value | – | <0.001 | 0.012 | <0.001 | |
| Elimination | Median (Q1, Q3) | 0 (0, 12) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 4533 | 5059 | 4015 | |
| P-value | – | <0.001 | 0.008 | <0.001 | |
| Yawning | Median (Q1, Q3) | 6 (0, 24) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| df | 110 | 110 | 110 | 110 | |
| Mann- Whitney (U) | – | 2709 | 2833 | 2585 | |
| P-value | – | <0.001 | <0.001 | <0.001 | |
Discussion
The vision of the horse due to its anatomical positioning of the eyes enables the horse to see completely on both sides of its body and nearly the entirety to the rear of their body (Timney & Keil, 1992). When a stimulus is present either auditory or visual is present in the adjacent fields the animal turns its head towards the stimulus to use the binocular field located down the nose. A very important fact about the binocular field of vision it is located down the nose of the horse, the horse has a frontal located blind spot, when the nose is drawn in and the face approaches the vertical the individual is unable to see directly in front, the field is described as ‘narrow’ (Harman et al., 1999). For distant objects, the horse would have to rotate the nose up high to observe the distant objects because the binocular overlap is oriented down the nose, with a blind area directly in front of the forehead. (Basile et al., 2009). Investigation of novel objects, horses tend to investigate by using only one eye for investigation (Des Roches et al., 2008). Visual lateralization has been demonstrated to be preferential in the left monocular field to monitor novel stimuli. This finding is supportive and consistent with left eye preferences found in numerous mammals, birds and amphibians responding to predators (Robins & Phillips, 2009). In our study the head-turning was significant during for duration and frequency this visual repetitive behaviour could be resulted from the horse switching to and from monocular and binocular vision. The movement of the head permits monocular fixation of the novel stimulus before a specific response/ reaction can be made (Rogers, 2017). Exposure to a sudden movement in stimulus caused more arousal behaviour with a higher increased heart rate response compared to horses exposed to a stationary novel stimulus (Christensen, 2006). In the study, a visual stimulus used was a red color cardboard a vibrant color to the human eye. Horses have a little sensitivity to color but more to movement, a horse’s vision is sensitive to dim light, rods influence colour perception at low light intensities (Saslow, 2002; Roth et al., 2008). This is due to the scotopic vision data has shown that the visual system of a horse is rod-dominated (Hanggi et al., 2009).
The investigative behaviour had increased in duration as earlier depicted with vigilance and head turning. However, unlike visual and olfactory stimulus, the auditory frequency had a lower mean frequency in the investigation. Results showed that a horse has large interaural distances and a broad binaural- localization cues available to the species. They have a poor hearing in contrast to other larger mammals with an average 22° for noise and 30° for clicks (Heffner & Heffner, 1984). The increased duration of the investigative behaviour is acknowledgment of the stimulus. Nevertheless, the low frequency of investigation can imply that the horse is unable to detect the source of the noise, therefore, the horse exhibits behaviours continuously. Heffner, (1998) states there are two factors that determine the aspects of a sound and if a horse will attend (1) adjacency to the stimulus, (2) novelty of the stimulus. Now in this study, we investigated the novelty of auditory stimulus we did not test the distance or the positioning of the source of the stimulus. The ability to recognise predator vocalisation and to react indicates a high sensitivity to predation (Delpietro, 1989), even though horses are limited in localising sound it is apparent that they can detect predation and other potential threatening stimulus. Vocalisation duration increased in the auditory experiment possible reason is due to the limited ability to localise the source of the stimulus, this inability to specify the region of the source causes a greater need to vocalise a possible threat to the rest of the herd. Early warning systems of predation such as one individual horse vocalising possible threats is an adaptive behaviour for survival, the domestication of horses has had little change in horse behaviour (Goodwin, 2007). This can answer the reason why vocalisation increased in the auditory experiment.
The study showed the olfactory stimulus bring about increased investigative behaviour in contrast to the control experiment, due to the behavioral parameter of sniffing and the Flehman response frequency, latency and duration are greater with the addition of olfactory stimuli (Marinier et al., 1988). Olfactory novel stimuli have been found in previous studies to have shown significant behavioural reactions in comparison to the control experiment, the only behavioural reaction not exhibited is the flight response, furthermore, an increase in heartrate was significantly increased (Ahmadinezhad et al., 2010). This increase in behaviour and not the flight response can be linked to the sexual and social behaviour. Living in complex social systems requires perceptual and cognitive capacities for recognition of group members, predators and probable sexual partners. Many animals can identify proteins in urine, skin secretions and saliva by scent this shows the importance of sniffing for olfactory recognition (Krueger & Flauger, 2011). The Flehmen response significantly increased in duration and frequency. Marinier et al., (1988) discovered a stallion’s response is much greater to an olfactory stimulus than mares and geldings which is supported by other species e,g, the relation between cows and bulls (Houpt et al., 1989). Stallions can differentiate the sex of another horse based on faecal matter alone but not off urine alone. This can be explanation of faecal marking behaviour within horses (Stahlbaum & Houpt 1989). Olfactory senses are used in the exchanging of information, among horses can implement contest before physical contact is necessary (Rubenstein & Hack, 1992). The increased Flehmen response can be linked into the increased frequency of tail posture as the unknown odour can result in hormonal response to cover the unknown scent with a horse’s own scent. Horses tend to use the olfactory stimulation to cause the animal to become more vigilant to their surroundings, whilst visual and auditory senses result in detection/ orientation towards danger (Christensen, 2006).
The results show that the ‘Vices’ duration were reduced slightly in visual and olfactory experiment, however, auditory experiment was similar to that of the control experiment. Though, the frequency of the category behaviour had decreased in all experiments in comparison to the control. Increasing the turn-out of horses that exhibit stereotypic ‘vice’ behaviour can reflect an ineffective way of treating horses with behavioural problems, additionally, reduction of forced exercise (Riding etc) is more likely to cause horses to exhibit behaviours (Luescher et al., 1998). This can possibly explain the low duration of the control experiment due to the horses in the study living conditions daily working or being out in paddocks instead of their stables. With the introduction of stimulus, a horse is given a potentially harmful stimulus or posed with a threat and if able to emotionally manage this is regarded as adaptation (Cooper & Albentosa, 2005). This can indicate why there wasn’t a considerable change in the duration of vice behaviour as control experiment can imply that the horses had behavioural issues or more likely the lack of mental stimulation. The introduction of novel stimuli can cause a horse to exhibit vice behaviour due to the potential harmful stress. After habituation training (form of learning that desensitizes to a stimulus after repeated presentation), the fear response is significantly reduced whereas the fear response remains for another novel object (Forkman et al., 2007). With habituation training can reduce vice behaviour within a horse but also result in mental stimulation if the novel object is not used to frequently or for long periods of time for the horse to become uninterested. There was significant reduction in the frequency of behaviours from the control to the novel stimuli experiments, this can indicator to an observer if the horse is unstimulated or exhibit vices due to the novel stimuli.
Frequency of behaviours as a majority decreased with the presence on environmental stimulation. Studies have shown in sows that dopamine systems are involved in selection and the commencement of motor activities, stereotyped behaviour are typically observed in situations of conflict/ frustration, with stereotyped behaviour increasing due to lack of environmental stimulation (Dantzer, 1986). Horse stereotype behaviour is stall- walking that what significantly reduced along with the vice category behaviours with the addition of stimulation. Further studies have supported these finding of novel stimuli resulting in low locomotion including Christensen et al., (2005). Low locomotion in the control experiment can be an indicative of negative emotions; boredom, frustration and aversion or even ill health such as pacing as well as the vice behaviour (Cooper & McGreevy, 2007). The introduction of novel stimuli reduces
Important to have knowledge of how a horse’s detection of a stimulus, perceived by the sensory organs and passes through different routes of the central nervous system (CNS) (Fig 3.).
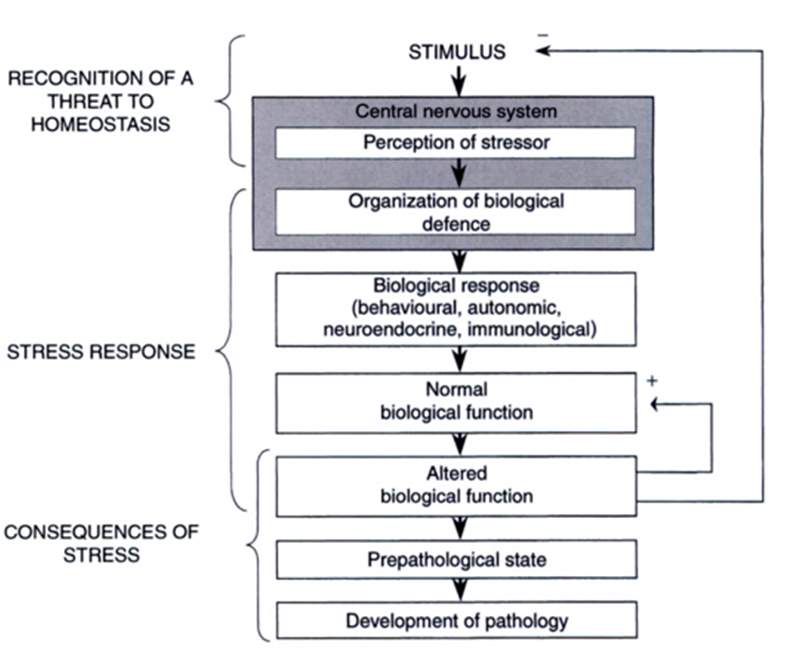
Figure 3. A model of the biological response (Taken from; Moberg & Mench, 2000))
Once a stimulus is detected the organisation of biological defence is organised with the cerebral cortex acting as a unit but has four localised regions in which sensory impulses are acknowledged and redirected; somasthetic area, visual area, auditory area and olfactory area (Fraser, 2010). Even though the mammalian cerebral cortex is not the only part of the brain in the understanding of the different sensory systems, clinical experience has argued it to be having a significant role in to redirect and organise the different senses (Jones & Powell, 1970).
Visual area
Visual stimulation is collected from the retina of the eye to the optic nerves and dispersed to the cortex visual area at the occipital part of the cerebrum. Clear recognition of situations with the selection of certain releasers active action patterns take place in the visual area (Fraser, 2010).
Auditory area
Located in the temporal lobe of the cerebral cortex and detects nerve impulses from the thalamus. The nerves of hearing end at the pons to the thalamus to finally the cortex (Fraser, 2010)
Olfactory area
Located in the hippocampus, which receives impulses from the centre of the olfactory bulbs, the centre deals with olfactory reflexes (Fraser, 2010).
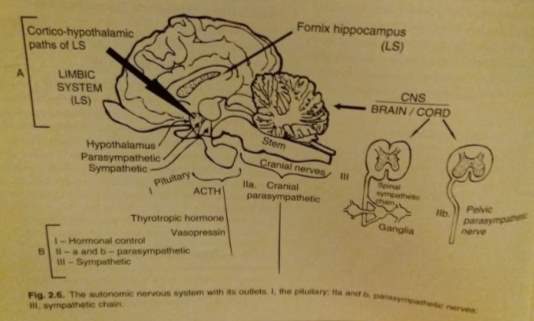
Figure 4. The autonomic nervous system (Taken from; Fraser, 2010)
The limbic system is vital for determining the correct strategic and tactical functions essential for adaptation and survival and the link between emotive and cognitive brain (Mogenson et al., 1980). It includes portions of the frontal lobe cortex, thalamus, temporal lobe and the hypothalamus with certain midbrain parts acting as a the function generators (Broad et al., 2002). The hypothalamus and cortex controls the peripheral autonomic system (control of the bodily functions) (MacLean, 1952). The impulses from the visual, olfactory and auditory sensory organs will be directed to the hypothalamus to result in a response to particular behavioural responses.
Limitations of the study are could be the time of the year we can not see if climate interfered with behaviours exhibited by the horses, further studies could see if there is any change between behaviours between the months of the year. Additionally, a greater sample size as in the study there was 10 individuals even though there was a high number of replicates, further investigation would include a greater sample size to see if the results are exclusive to the horses observed within this study. A greater sample size, increase the precision of the data, reduce the margin of error thus enabling to identify any outliers that may occur. There are numerous factors that can affect a behavioural study such as whether conditions, dietary, exercise, degrees of emotionality. Natural science studies will always face these limitations dealing with living organisms, numerous experiments have to be undertaken with varying degrees of aspect to cement one theory with several replicates of the experiment completed. Nevertheless, can these results be expected in all horses or would the results from this method differ between horses in other sports; dressage jumping racing? Previous studies of behavioural variables across test stimulations on horses have shown varying results examples are shown in these studies to what extant results can vary; Scholen et al., 1997; Wolff et al., 1997; Visser et al., 2001; Seaman et al., 2002.
To conclude a horse uses their monocular vision to identify novel stimulus and further aspects the unknown environmental stimulation by using its binocular vision, additionally, the horse’s vision is far better at low intensities of light in comparison to high- intensities. The horse’s hearing is limited with the inability to localise where the source of auditory stimulation is from. Thus, leading to vocalisation, probably due to their evolutionary history of being prey to predators. Olfactory response results in pheromonal behavioural response for a sexual or social competition. Vice/ stereotyped behaviour is caused by a lack of environmental stimulation or by the unknown stimulus that may possibly cause harm. A way of depicting the cause is due to the frequency low frequency indicates a novel stimulus whilst the higher frequency indicates a lack of stimulation. The cerebral cortex organises sensory impulses and results in the appropriate autonomic and behavioural response. This study has identified the various behaviours exhibited by horses and there is a further understanding of how a horse would react to a novel stimulus, this understanding can reduce unwanted behaviours within a stalled horse safety for both horse and handler. Additionally, the welfare of the horse can be increased by understanding the value of mental stimulation alongside the easier identification of health implications that a horse can possibly express. Further studies can look into the strength of each stimulus and how that further effects the behavioural responses to a horse.
References
AHMADINEZHAD, M., HASANI, A., & KHARAZIAN, F. (2010). The responses of horses to predator stimuli.
Austin, N. P., & Rogers, L. J. (2012). Limb preferences and lateralization of aggression, reactivity and vigilance in feral horses, Equus caballus. Animal behaviour, 83(1), 239-247.
Austin, N. P., & Rogers, L. J. (2014). Lateralization of agonistic and vigilance responses in Przewalski horses (Equus przewalskii). Applied Animal Behaviour Science, 151, 43-50.
Basile, M., Boivin, S., Boutin, A., Blois-Heulin, C., Hausberger, M., & Lemasson, A. (2009). Socially dependent auditory laterality in domestic horses (Equus caballus). Animal cognition, 12(4), 611-619.
Broad, K. D., Mimmack, M. L., Keverne, E. B., & Kendrick, K. M. (2002). Increased BDNF and trk‐B mRNA expression in cortical and limbic regions following formation of a social recognition memory. European Journal of Neuroscience, 16(11), 2166-2174.
Christensen, J. W., Keeling, L. J., & Nielsen, B. L. (2005). Responses of horses to novel visual, olfactory and auditory stimuli. Applied Animal Behaviour Science, 93(1), 53-65.
Christensen, J. W. (2006). Fear in horses (Vol. 2006).
Clutton-Brock, J. (1999). A natural history of domesticated mammals. Cambridge University Press.
Cooper, J. J., & MASON, G. J. (1998). The identification of abnormal behaviour and behavioural problems in stabled horses and their relationship to horse welfare: a comparative review. Equine Veterinary Journal, 30(S27), 5-9.
Cooper, J. J., & Albentosa, M. J. (2005). Behavioural adaptation in the domestic horse: potential role of apparently abnormal responses including stereotypic behaviour. Livestock production science, 92(2), 177-182.
Cooper, J., & McGreevy, P. (2007). Stereotypic behaviour in the stabled horse: causes, effects and prevention without compromising horse welfare. In The welfare of horses (pp. 99-124). Springer, Dordrecht.
Dantzer, R. (1986). Symposium on “Indices to Measure Animal Well-Being” Behavioral, Physiological and Functional Aspects of Stereotyped Behavior: A Review and a Re-Interpretation. Journal of Animal Science, 62(6), 1776-1786.
Delpietro, H. A. (1989). Case reports on defensive behaviour in equine and bovine subjects in response to vocalization of the common vampire bat (Desmodus rotundus). Applied Animal Behaviour Science, 22(3), 377-380.
Des Roches, A. D. B., Richard-Yris, M. A., Henry, S., Ezzaouïa, M., & Hausberger, M. (2008). Laterality and emotions: visual laterality in the domestic horse (Equus caballus) differs with objects’ emotional value. Physiology & Behavior, 94(3), 487-490.
Evans, J. W. (2000). Horses: a guide to selection, care, and enjoyment. Henry Holt and Company.
Forkman, B., Boissy, A., Meunier-Salaün, M. C., Canali, E., & Jones, R. B. (2007). A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiology & Behavior, 92(3), 340-374.
Fraser, A. F. (2010). The behaviour and welfare of the horse. CABI.
Goodwin, D. (2007). Horse behaviour: evolution, domestication and feralisation. In The welfare of horses (pp. 1-18). Springer, Dordrecht.
Hanggi, E. B., & Ingersoll, J. F. (2009). Stimulus discrimination by horses under scotopic conditions. Behavioural processes, 82(1), 45-50.
Harman, A. M., Moore, S., Hoskins, R., & Keller, P. (1999). Horse vision and an explanation for the visual behaviour originally explained by the ‘ramp retina’. Equine veterinary journal, 31(5), 384-390.
Heffner, H. E., & Heffner, R. S. (1984). Sound localization in large mammals: localization of complex sounds by horses. Behavioral neuroscience, 98(3), 541.
Heffner, H. E. (1998). Auditory awareness. Applied Animal Behaviour Science, 57(3), 259-268.
Houpt, K. A., Rivera, W., & Glickstein, L. (1989). The flehmen response of bulls and cows. Theriogenology, 32(3), 343-350.
Jones, E. G., & Powell, T. P. S. (1970). An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain, 93(4), 793-820.
Krueger, K., & Flauger, B. (2011). Olfactory recognition of individual competitors by means of faeces in horse (Equus caballus). Animal cognition, 14(2), 245-257.
Le Scolan, N., Hausberger, M., & Wolff, A. (1997). Stability over situations in temperamental traits of horses as revealed by experimental and scoring approaches. Behavioural processes, 41(3), 257-266.
Luescher, U. A., McKeown, D. B., & DEAN, H. (1998). A cross‐sectional study on compulsive behaviour (stable vices) in horses. Equine veterinary journal, 30(S27), 14-18.
MacLean, P. D. (1952). Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain). Electroencephalography and clinical neurophysiology, 4(4), 407-418.
Marinier, S. L., Alexander, A. J., & Waring, G. H. (1988). Flehmen behaviour in the domestic horse: discrimination of conspecific odours. Applied Animal Behaviour Science, 19(3-4), 227-237.
McGreevy, P., & Nicol, C. (1998). Physiological and behavioral consequences associated with short-term prevention of crib-biting in horses. Physiology & behavior, 65(1), 15-23.
McGreevy, P. D., & McLean, A. (2005). Behavioural problems with the ridden horse. The domestic horse, the origins, development and management of its behaviour. Cambridge University Press, Cambridge, 196-211.
Moberg, G. P., & Mench, J. A. (Eds.). (2000). The biology of animal stress: basic principles and implications for animal welfare. CABI.
Mogenson, G. J., Jones, D. L., & Yim, C. Y. (1980). From motivation to action: functional interface between the limbic system and the motor system. Progress in neurobiology, 14(2-3), 69-97.
Nicol, C. (1999). Understanding equine stereotypies. Equine veterinary journal, 31(S28), 20-25.
Robins, A., & Phillips, C. (2010). Lateralised visual processing in domestic cattle herds responding to novel and familiar stimuli. Laterality, 15(5), 514-534.
Rogers, L. J. (2017). Eye and ear preferences. In Lateralized Brain Functions (pp. 79-102). Humana Press, New York, NY.
Roth, L. S., Balkenius, A., & Kelber, A. (2008). The absolute threshold of colour vision in the horse. PLoS One, 3(11), e3711.
Rubenstein, D. I., & Hack, M. A. (1992). Horse signals: the sounds and scents of fury. Evolutionary Ecology, 6(3), 254-260.
Saslow, C. A. (2002). Understanding the perceptual world of horses. Applied Animal Behaviour Science, 78(2), 209-224.
Seaman, S. C., Davidson, H. P. B., & Waran, N. K. (2002). How reliable is temperament assessment in the domestic horse (Equus caballus)?. Applied Animal Behaviour Science, 78(2), 175-191.
Stahlbaum, C. C., & Houpt, K. A. (1989). The role of the flehmen response in the behavioral repertoire of the stallion. Physiology & behavior, 45(6), 1207-1214.
Timney, B., & Keil, K. (1992). Visual acuity in the horse. Vision Research, 32(12), 2289-2293.
Visser, E. K., Van Reenen, C. G., Van der Werf, J. T. N., Schilder, M. B. H., Knaap, J. H., Barneveld, A., & Blokhuis, H. J. (2002). Heart rate and heart rate variability during a novel object test and a handling test in young horses. Physiology & Behavior, 76(2), 289-296.
Wolff, A., Hausberger, M., & Le Scolan, N. (1997). Experimental tests to assess emotionality in horses. Behavioural processes, 40(3), 209-221.
Zeuner, F. E. (1963). A history of domesticated animals. A history of domesticated animals.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Animal Sciences"
Animal science can be described as studying the biochemistry, physiology, and behaviour of animals that are under human control. Historically, animal science degrees were known as animal husbandry and focused on livestock. Studies now include companion animals such as cats and dogs.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




