Acute Viral Gastroenteritis Overview and Case Study
Info: 6333 words (25 pages) Dissertation
Published: 2nd Sep 2021
Introduction
Acute gastroenteritis, commonly known as infectious diarrhoea, is the inflammation of the gastrointestinal tract, which can result in vomiting, abdominal pain and persistent diarrhoea. Gastroenteritis has consistently been one of the most prevalent illnesses affecting the global population throughout history, especially in infants and the elderly. In less developed countries such as: West African nations, gastroenteritis is a primary cause of death for infants under the age of five. In more developed countries, majority of infant cases are not fatal. However, they do result in hospitalisation or medical center visits, at a significant cost to the respective local economies (Chadwick and Goode, 2010). It is rare for individuals that live beyond infancy to have not been infected or suffered symptoms of viral gastroenteritis (Das et al., 2014). Ever since the 1940’s, viruses have been considered to be a prevalent cause of gastroenteritis, although at this time the etiology was relatively unknown in the majority of reported cases. Until Kapikian et al. identified the Norwalk virus within faeces during a diarrhea outbreak in 1972, which was shown to be a cause of gastroenteritis (Wilhelmi et al, 2003). With further observations such as Bishop et al., identifying rotavirus in the duodenal mucosa in children diagnosed with acute gastroenteritis in 1973. The research continued in 1975 with Flewett et al. publishing a paper in The Lancet with the identification of enteric adenoviruses in the faeces of patients in a long-term children’s ward. More recently, viruses known to cause diarrhoea in animals have shown to be associated with acute gastroenteritis in humans; these include coronavirus (Caul et al, 1975), picobirnaviruses, pestiviruses and toroviruses.
Aetiology
Rotaviruses are members of the family Reoviridae, they are characterised by a non-enveloped multi-layered virion 70-75 nm in length and 11-segmented double stranded RNA. Up to now nine, possibly ten species of rotavirus have been identified, which are referred to as rotavirus A to J (Matthijnssens et al., 2012). Only rotavirus A, B, C, and H are pathogenic to humans with A being the most medically significant of the species. The length of rotavirus genomes are estimated to be 18.5 kb pairs, with 11 or 12 possible proteins encoded, these include six structural proteins referred as VP1-4, 6 and 7, as well as 5 non-structural proteins, NSP1-5. VP4 and 7 are surface proteins involved in cell attachment and they have been shown to elicit the production of neutralising antibodies. Sporadic detection of rotavirus strains commonly associated with animals indicate some extent of permeability in host-specific barriers, with human strains such as G5, 8, 9 and 12 hypothesised to have animal host origins (Dóró et al., 2015).
Epidemiology
Acute Viral Gastroenteritis is one of the most prevalent viral infections in the world today, with a study by Scallen et al., estimating that in the four developed countries: Australia, Canada, Ireland and the USA, the cases of diarrheal diseases range within 0.44 and 0.99 cases per person per year. There are five common rotavirus genotypes responsible for the majority of rotavirus gastroenteritis cases, which are Wa-like G1P[8], G3P[8], G4P[8], G9P[8] and the DS-1-like G2P[4]. With 75% of all strains genotyped between 1996 and 2007, prior to extensive rotavirus vaccination in the majority of countries, belonging to one of these five strains. (Bányai et al., 2012). Many factors affect norovirus genotypes such as seasons, geographical location and years, with cold winter months seeing a rise in outbreaks (Ahmed et al., 2013). Suggestion for this correlation is due to an increase in particle stability at low humidity (below 0.007kg/kg air). Which could explain the rise in norovirus-induced illness during the winter months where humidity is constantly low. (Colas de la noue et al., 2014). Epidemiological surveys have shown a notable diversity and unpredictability of norovirus genotype distribution across the globe, showing ubiquitous distribution of norovirus throughout multiple environmental studies (Fernandez et al., 2012). With most clinical surveys observing two distinct norovirus features which are the predominance of GII viruses compared with GI, and the relatively higher prevalence of the genotype GII.4 (Mathews et al., 2012). Dissimilarly, GI prominence was found in environmental studies of shellfish cases and waterborne outbreaks. Although rotavirus- induced gastroenteritis shows consistent seasonality in wealthier countries with increased cases in winter, such cases are less seasonal in more underdeveloped countries, indicating the increased exposure of rotavirus to the population these poorer nations, with infrastructural factors likely to be influential in such results (Patel et al., 2013).
The majority of norovirus capsid proteins interact with intestinal HBGA carbohydrate ligands (Marionneau et al., 2002), with a variety of intestinal HBGA molecules and glycan-based molecules, for example galactosylceramide that the norovirus could possibly bind to (Bally et al., 2012). However, a definitive mechanism showing how norovirus enters a host cell is yet to be established. Inherited alleles determine an individual’s HBGA phenotype and the manner of HBDA antigen presentation, which subsequently determines norovirus binding ability and thus, the individual’s norovirus-induced disease susceptibility. Due to such factors norovirus susceptibility can differ greatly in different demographics (Currier et al., 2015).
Clinical Symptoms and Sickness Response
In humans, rotavirus-induced gastroenteritis has a variety of enteric responses, which can be asymptomatic or can vary between mild, severe or fatal symptoms often causing dehydration. The incubation period of rotavirus diseases is generally below 48 hours including sudden onset of sustained symptoms such as vomiting and diarrhea, which can last up to 5-6 days (Uhnoo et al., 1986). However, the symptoms of rotavirus-induced infection are not confined to life threating vomiting and diarrhea, they an array of systemic responses referred to as “sickness responses”, which details the initial response of the immune system to a variety of potential pathogenic agents, also referred to as the acute phase response. Unlike many bacterial gastrointestinal infections, two biomarkers of inflammation CRP and calprotectin levels remain constant in a RV infection. However, the inflammatory reaction is mediated by interleukin-1 and IL-6, as well as alpha (TNF-α) which is a tumour necrosis factor. Studies show that the most severe inflammatory responses such as prolonged diarrhea, leukocytosis and bloody stool are associated with bacterial pathogens, suggesting norovirus-induced gastroenteritis, although still producing symptoms such as nausea and vomiting, have a decreased severity of symptoms comparatively to bacterial-induced gastroenteritis.
Other acute-phase responses are triggered by norovirus, such as fever, which derives from the hypothalamus and part of the body’s initial immune response (Brodal, 2010). Fever is commonly accompanied by other typical disease symptoms such as fatigue, depression, sleepiness loss of appetite and thirst.
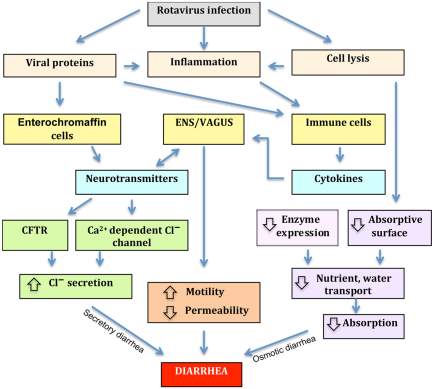
Figure 1.Four mechanisms associated with rotavirus-induced diarrhea; osmotic diarrhea, altered motility, secretory diarrhea and permeability.
Assuming rotavirus-induced diarrhea includes one or more of these mechanisms is reasonable. Osmotic diarrhea can be cause by high concentrations of inefficiently absorbable compound as this can produce an osmotic gradient within the intestine, causing the flow of water across the intestinal epithelium. While other diarrhea mechanisms such as secretory diarrhea stem the secretory capacity of the intestinal tract being overstimulated by nerve cells, separate to a mechanism responsible for the inhibition of fluid absorption. Another mechanism causes water and electrolytes to accumulate in the lumen as the barrier function of the epithelium is compromised due to the disruption of tight junctions via hydrostatic pressure in lymphatic’s and blood vessels. Excitatory and inhibitory signals from the ENS and CNS influence the motility of the small intestine as well as the entire digestive tract, with loperamide reducing motility within the intestine via opioid receptors within the myenteric plexa. Many observations have shown intestinal permeability to be controlled by the vagus nerve, suggesting rotavirus-induced gastroenteritis disease mechanisms are not limited to vomiting, electrolyte secretion and intestinal mobility, but may also include intestinal permeability (Constantini et al., 2010). Figure source (Michelangeli and Ruiz, 2003).
Pathogenesis
Focusing on rotavirus-induced gastroenteritis, which causes approximately 450,000 deaths globally each year, most of which occur in emergent countries and mainly in children of under 5 years old (Tate et al., 2012). A range of factors, both viral and host influence if the disease is symptomatic or asymptomatic with age being a prevalent factor, in neonatal cases patients rarely expressing the disease symptomatically. The main reason for this is thought to be the transplacental transfer of maternal antibodies (Ray et al., 2007). Such antibodies reduce as the newborn ages, which correlates to the age of maximum susceptibility of children to rotavirus-induced gastroenteritis.
Rotaviruses cause infection in mature enterocytes found on the tips of the villi found in the small intestine, which causes epithelium atrophy in the villi. The mechanism in which diarrhea is produced are multifactorial and begin when rotaviruses attach to and infect the intestinal enterocytes. Rotavirus binding is mediated by the interaction of multiple sialic acid- containing and nonsialylated receptor molecules, once internalized viral RNAs and proteins concentrate within viroplasms located within the enterocytes cytoplasm, this is where replication and packing of viral RNA is performed. Intracellular mechanisms involving the viral protein NSP4 triggers the release of calcium ions from the endoplasmic reticulum, which in turn causes the disruption of the microvilli cytoskeletal network and decrease in absorption of the villous epithelium in relation to the crypt cells secretion capacity (Ramig, 2004). Subsequently intestinal permeability is decreased to molecules such as lactose, as well as a decreased number of intestinal disaccharidase. The virus triggers an enteric nervous system, which causes increased electrolyte secretion and water induction within the small intestine (Lundgren et al, 2000). Additionally, a viral protein NSP4, in whole form, fragmented or certain NSP4 peptides have exhibited toxic effects and has shown to induce diarrhea in mice (Zhang et al., 2000). A calcium dependent secretion pathway prior to cell lysis specifically mediates the release of NSP4, which leads to malabsorption and the reduction of digestive enzyme expression on the surface of the epithelial cell. NSP4 binds to cells using specific yet unknown receptors and initiates a (PLC-IP3) influx that results calcium ions being released from the endoplasmic reticulum, increasing the concentration of calcium within the cell. When NSP4 acts on enterocytes, there is disruption of tight junction, which causes paracellular permeability. When NSP4 acts on crypt cells, secretion is caused by the increase in calcium concentration and is mediated by the activation of a chloride transporter. This is responsible for the increased secretory component of the diarrhea.
Multiple studies support that local intestinal immunity prevents the occurrence of multiple incidences of diarrhea in succession. Gorell et al. established that only the primary infection of noroviruses elicited a homotypic neutralising antibody response, the any subsequent infections eliciting heterotypic responses. With research, showing that protein NSP4 is responsible for producing a cellular immunity-related response (Johansen et al, 1999).
Diagnostic Strategy
All viruses found to definitively cause gastroenteritis have been successfully grown in cell culture, with the exception of with the exception of human caliciviruses. With the ability to cultivate these viruses leading to the production of reagents that can be used in diagnostic studies, allowing a better understating of factors in regards to immunity of infection and a clearer understanding of the virus’s life cycle. The first example of diagnosis of acute viral gastroenteritis was in 1972, when Kapikian et al. identified 27nm viral particles in stool samples used to induce gastroenteritis in human volunteers collected from an outbreak of acute gastroenteritis in Norwalk, Ohio in 1968, using immune electron microscopy. During further studies, other small round-structured viruses, referred to as SRSVs were morphologically similar to Norwalk virus and also induced outbreaks of acute gastroenteritis (Atmar and Estes, 2001). Initially gastroenteritis-inducing viruses were classified purely based on morphology, however with advances in molecular biology, viruses are classified based on specific genomic characteristics, with most SRSVs being characterised as Caliciviridae.
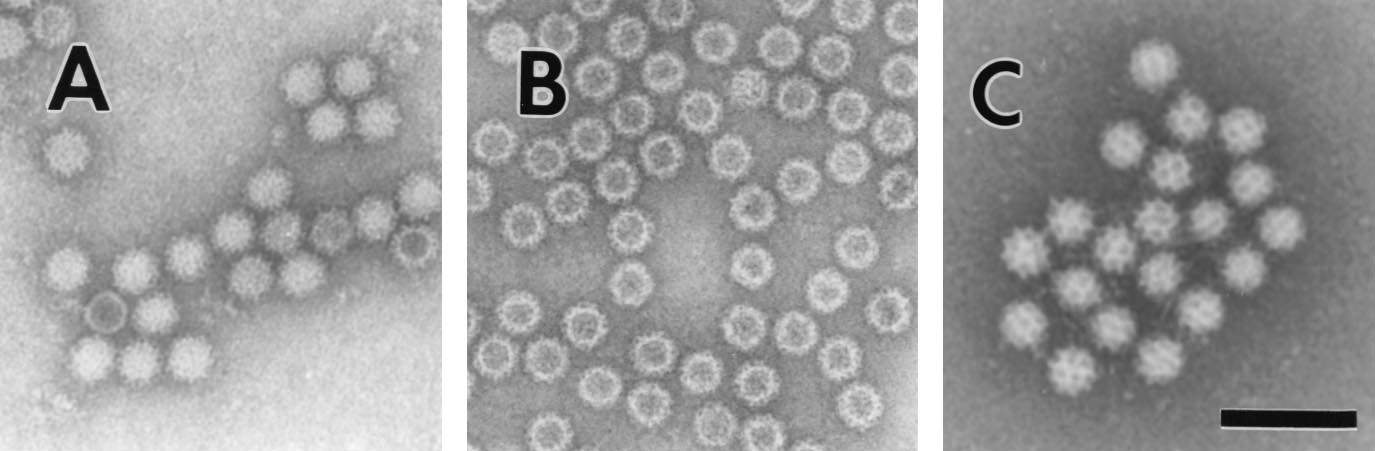
Figure 2. Electron micrographs of (A) Norwalk Virus, (B) baculovirus-expressed NVL particles, and (C) Sapporo virus. Bar, 100 nm (Atmar and Estes, 2001).
However, these primitive techniques have relatively limited sensitivity with at least 106 viral particles per ml of stool being required for detection to be accurate, as well as being labour intensive (van Maarseveen et al., 2010). The preferred diagnostic method for microbiology laboratories commercial antigen detection kits such as latex agglutination and enzyme immunoassays, as these kits have a great level of specificity. However due to the diverse nature of gastrointestinal viruses, the estimated sensitivity for such kits is relatively poor. Recent diagnosis for gastroenteritis-inducing viruses has switched to real-time PCR assays, as they offer significantly improve sensitivity and specificity compared to immune electron microscopy and antibody-detection assays, with a reported 48% increase in detection rate using reverse transcription-PCR compared to electron microscopy or EIA (Catriona et al., 2006). Although a vast improvement on earlier methods of testing, these methods have their limitation substantial time consumption and many only having the capacity to test for one virus per assay (Dunbar, 2013). This most recent diagnostic technique implemented is multiplex molecular assays which allow for the simultaneous detection of a variety of gastroenteritis-inducing pathogens, not just specific gastrointestinal viruses, meaning the turnaround time to produce sufficiently accurate results is decreased while additionally identifying distinct infection previously undetectable by single pathogen testing methods. Recent testing of three commercial multiplex detection platforms by Chhabra et al. in 2017, showed overall sufficient sensitivity and specificity, of such platforms was >90% when inoculated with a sample mixtures positive for one or more of five gastroenteritis-inducing viruses. However, the sensitivity towards specific viruses is relatively low, possible factors for such results are the difficulty for the platforms to successfully detect low viral load samples. These multiplex enteric pathogen platforms are increasing in popularity with more than 15% of diagnostic laboratories utilizing these platforms to in the detection and characterisation of pathogens (Hurd et al., 2012). The most advanced diagnostic apparatus for the detection and characterisation of gastroenteritis include Filmarray GI panel, Enteric pathogens (EP) test Nanospheres verigene, and CLART EnteroBac (Reddington et al., 2014)
Treatment
The various symptoms of gastroenteritis involve the expulsion and loss of fluid from the body in the form of vomiting and diarrhea with varying severity, with this in mind and the relatively short duration period of one to two weeks. Subsequently rehydration has long been the main method of treatment and management of acute viral gastroenteritis regardless of severity, with the first example of dehydration treatment during cholera epidemics in the 1830’s. Over a century later, during the 1050’s, intravenous fluid treatment was used as a treatment for cholera-induced dehydration. Due to apparent complications in locating suitable veins in dehydrated patients and infection issue within poorly funded facilities where most cases occurred, intravenous fluid treatment was seen to be insufficient and in the late 1960’s and early 70’s efforts were made to manufacture cheap and effective oral rehydration solutions (ORS) (Svensson et al., 2016).
The need for rehydration stems from the net loss of body water experienced during prolonged periods of diarrhea, with the imbalance of absorption and secretion causing up to several litres of lost body water per day. Accompanying the water loss is the loss of sodium and other electrolytes almost exclusively found in blood plasma and other bodily fluids (Field. 2003). With prolonged periods of diarrhea comes rapid depletion of body water and sodium; intestinal absorption occurs via osmosis meaning if sodium ions are not absorbed into the intestine, water will not be absorbed either. With oral rehydration being the main mechanism for sodium absorption, it is the most effective method of sodium replenishment and body water retention. This is important as most gastroenteritis-induced deaths are cause by severe dehydration, as a net loss of over 10% of body fluids can cause fatality.
Table 1. List of ingredients and proportions in oral rehydration solutions (WHO Drug Information Vol. 16, No. 2, 2002.)
| Reduced osmolarity ORS | grams/litre | Reduced osmolarity ORS | mmol/litre |
| Sodium chloride | 2.6 | Sodium | 75 |
| Glucose, anhydrous | 13.5 | Chloride | 65 |
| Potassium chloride | 1.5 | Glucose, anhydrous | 75 |
| Trisodium citrate | Potassium | 20 | |
| dihydrate | 2.9 | Citrate | 10 |
| Total Osmolarity | 245 |
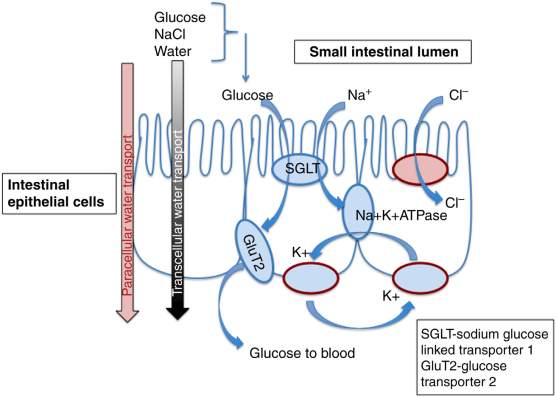
Figure 3. Representation of oral rehydration mechanisms (Svensson et al., 2016)
Prevention
Total number diarrhea related fatalities have steadily dropped over time; this is due to a combination of different factors including improved oral rehydration therapy, improvements in general sanitation, hygiene, and the promotion of breastfeeding. However, viral gastroenteritis fatality rates have declined at a much slower rate as these factors have significantly less effect. Vaccines for rotavirus-induced gastroenteritis is available in three categories, attenuated human rotavirus strains, animal-human reassortant rotavirus strains and heterologous animal rotavirus strains. The vaccine work by triggering immune responses consistent with those triggered by a rotavirus infection, such as the production of RV serum IgA antibodies within the patient. Studies have shown correlation between a high titre of RV serum IgA antibodies and protection against rotavirus infections (Ward et al., 1992). These antibodies bind to rotavirus surface antigen VP6, aiding the neutralisation of subsequent rotavirus infections. However, Anti-VP6 antibodies are classified and being nonneutralising as they do not prevent in vitro or in vivo rotavirus infections (Feng et al., 2002). Studies show that anti-VP6 IgA antibodies may protect against rotavirus diseases at a later stage (Corthésy et al, 2006). Aiyegbo et al. suggest anti-VP6 antibodies prevent the release of rotavirus RNA by stabilising the VP6 inner core particle, subsequently protecting from rotavirus-induced diseases.
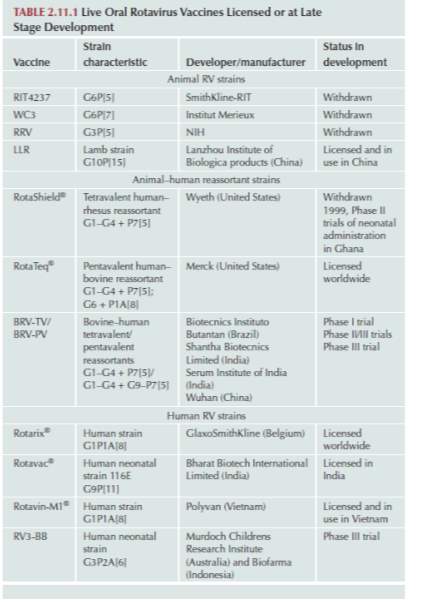
Table 2. Current Licensed or late development Oral Rotavirus Vaccines (Svensson et al., 2016)
Case Study
In 2012, a 20-week-old female was admitted into hospital with 48 hours of alleged diarrhea and a fever, which had developed within 12 hours of admission. In the previous 24 hours the infant had reportedly produce 10 watery stools and had grown unsettled, persistently crying and reduced fluid intake of 50%, there was no record of vomiting.
An enquiry into the infant’s medical records revealed birth via emergency C-section with a birth weight of 2.91kg. She was placed into an induced coma and active hypothermia for her first 72 hours of life. Post discharge, until the current hospitalisation, she did not receive any vaccines, and her weight was 4.39kg two weeks prior to current admission.
Table 3. Results of initial testing on the day of admission (Guzganu, 2012).
| Test | Result |
| Internal Temperature (0C) | 39.9 |
| Heart Rate (Beats/Minute) | 170-190 |
| Respiratory Rate (Breaths/Minute) | 40-80 |
| Blood Pressure (mmHg) | 102/55 |
| Oxygen Saturation by Pulse Oximetry (%) | 100 |
| Admission Weight (Kg) | 3.99 |
The infants admission weight was concerning as it showed a 10% decrease compared to her weight 2 weeks prior. Her skin was a pale grey, had dry lips and buccal mucosa, reduced tears, and 3-second capillary refill. Urine output was significantly decreased, with abdominal swelling. On completion of routine analysis of a stool sample, rotavirus antigen was identified, with urine and blood culture testing producing negative results.
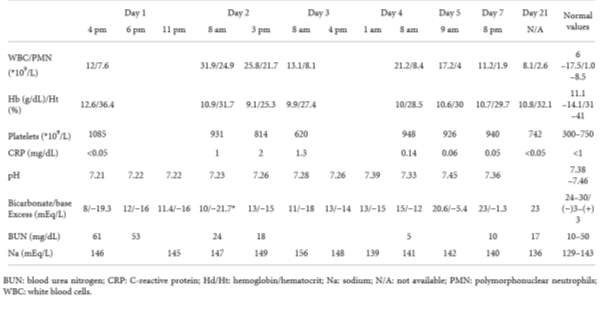
Table 4. Laboratory result for duration of hospitalisation (Guzganu, 2012).
To correct the child’s low blood pH, tachycardia and polypnea, fluid therapy was administered intravenously, with two doses of volume repletion solution containing 0.9% NaCl at 20 mL/kg over an hour period. Soon after, a 5% dextrose solution was administered to assist in gradual hypernatremia correction, and within four hours of rehydration therapy diuresis was successfully induced. The patient received oral rehydration solution in an effort to replenish digestive loss of water and electrolytes with an initial does of 10mL/kg, which was subsequently replaced volume by volume subject to net stool loss. Enteral nutrition was then achieved through lactose-free formula milk (Novalac Diarinova).
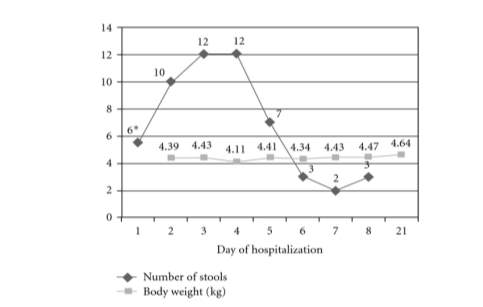
Figure 4. Evolution of daily stool count and body weight throughout hospitalisation (Guzganu, 2012).
*= Stool count from time of admission (12pm)
Future Developments
Due to gastroenteritis’s unprecedented disease burden, a significant emphasis was put on the development of vaccines for the multiple pathogenic causes. Finally, after clinical trials spanning 30 years, rotavirus vaccines are now available in over 100 countries, with possible norovirus candidates in development. However, preliminary data has indicates the genetic and antigenic diversity of noroviruses will hinder the development of such vaccines, preventing the realisation of cross-protective clinical immunity (Bányai et al., 2018)
References
Bányai, K., Estes, M.K., Martella, V. and Parashar, U.D., 2018. Viral gastroenteritis. The Lancet.
Chadwick, D. and Goode, J. (2010). Gastroenteritis viruses. 1st ed. Chichester, West Sussex: Wiley, pp.5-25.
Dóró, R., Farkas, S.L., Martella, V. and Bányai, K., 2015. Zoonotic transmission of rotavirus: surveillance and control. Expert review of anti-infective therapy, 13(11), pp.1337-1350.
Majowicz, S., Musto, J., Scallan, E., Angulo, F., Kirk, M., O’Brien, S., Jones, T., Fazil, A. and Hoekstra, R. (2010). The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clinical Infectious Diseases, 50(6), pp.882-889.
Scallan, E., Majowicz, S.E., Hall, G., Banerjee, A., Bowman, C.L., Daly, L., Jones, T., Kirk, M.D., Fitzgerald, M. and Angulo, F.J., 2005. Prevalence of diarrhoea in the community in Australia, Canada, Ireland, and the United States. International journal of epidemiology, 34(2), pp.454-460.
Wilhelmi, I., Roman, E. and Sanchez-Fauquier, A., 2003. Viruses causing gastroenteritis. Clinical microbiology and infection, 9(4), pp.247-262.
Kapikian, A.Z., 1996. Overview of viral gastroenteritis. In Viral Gastroenteritis (pp. 7-19). Springer, Vienna.
Caul, E.O., Paver, W.K. and Clarke, S.K.R., 1975. Coronavirus particles in faeces from patients with gastroenteritis. The Lancet, 305(7917), p.1192.
Lundgren, O., Peregrin, A.T., Persson, K., Kordasti, S., Uhnoo, I. and Svensson, L., 2000. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science, 287(5452), pp.491-495.
Gorrell, R.J. and Bishop, R.F., 1999. Homotypic and heterotypic serum neutralizing antibody response to rotavirus proteins following natural primary infection and reinfection in children. Journal of medical virology, 57(2), pp.204-211.
Johansen, K., Hinkula, J., Espinoza, F., Levi, M., Zeng, C., Rudén, U., Vesikari, T., Estes, M. and Svensson, L., 1999. Humoral and cell‐mediated immune responses in humans to the NSP4 enterotoxin of rotavirus. Journal of medical virology, 59(3), pp.369-377.
Phillips, A.D. and Rice, S.J., 1982, January. ASTROVIRUS WITHIN HUMAN SMALL INTESTINAL-MUCOSA. In Gut (Vol. 23, No. 10, pp. A923-A924). BRITISH MED ASSOC HOUSE, TAVISTOCK SQUARE, LONDON, ENGLAND WC1H 9JR: BRITISH MED JOURNAL PUBL GROUP.
Midthun, K., Greenberg, H.B., Kurtz, J.B., Gary, G.W., Lin, F.Y. and Kapikian, A.Z., 1993. Characterization and seroepidemiology of a type 5 astrovirus associated with an outbreak of gastroenteritis in Marin County, California. Journal of clinical microbiology, 31(4), pp.955-962.
Tate, J.E., Burton, A.H., Boschi-Pinto, C., Steele, A.D., Duque, J. and Parashar, U.D., 2012. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. The Lancet infectious diseases, 12(2), pp.136-141.
Ray, P.G., Kelkar, S.D., Walimbe, A.M., Biniwale, V. and Mehendale, S., 2007. Rotavirus immunoglobulin levels among Indian mothers of two socio‐economic groups and occurrence of rotavirus infections among their infants up to six months. Journal of medical virology, 79(3), pp.341-349.
Zhang, M., Zeng, C.Q.Y., Morris, A.P. and Estes, M.K., 2000. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells. Journal of Virology, 74(24), pp.11663-11670.
Ramig, R.F., 2004. Pathogenesis of intestinal and systemic rotavirus infection. Journal of virology, 78(19), pp.10213-10220.
Bányai, K., László, B., Duque, J., Steele, A.D., Nelson, E.A.S., Gentsch, J.R. and Parashar, U.D., 2012. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine, 30, pp.A122-A130.
Ahmed, S.M., Lopman, B.A. and Levy, K., 2013. A systematic review and meta-analysis of the global seasonality of norovirus. PloS one, 8(10), p.e75922.
de la Noue, A.C., Estienney, M., Aho, S., Perrier-Cornet, J.M., de Rougemont, A., Pothier, P., Gervais, P. and Belliot, G., 2014. Absolute humidity influences the seasonal persistence and infectivity of human norovirus. Applied and environmental microbiology, pp.AEM-01871.
Fernández, M.D.B., Torres, C., Poma, H.R., Riviello-López, G., Martínez, L.C., Cisterna, D.M., Rajal, V.B., Nates, S.V. and Mbayed, V.A., 2012. Environmental surveillance of norovirus in Argentina revealed distinct viral diversity patterns, seasonality and spatio-temporal diffusion processes. Science of the Total Environment, 437, pp.262-269.
Matthews, J.E., Dickey, B.W., Miller, R.D., Felzer, J.R., Dawson, B.P., Lee, A.S., Rocks, J.J., Kiel, J., Montes, J.S., Moe, C.L. and Eisenberg, J.N.S., 2012. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiology & Infection, 140(7), pp.1161-1172.
Marionneau, S., Ruvoën, N., Le Moullac–Vaidye, B., Clement, M., Cailleau–Thomas, A., Ruiz–Palacois, G., Huang, P., Jiang, X. and Le Pendu, J., 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology, 122(7), pp.1967-1977.
Bally, M., Rydell, G.E., Zahn, R., Nasir, W., Eggeling, C., Breimer, M.E., Svensson, L., Höök, F. and Larson, G., 2012. Norovirus GII. 4 Virus‐like Particles Recognize Galactosylceramides in Domains of Planar Supported Lipid Bilayers. Angewandte Chemie International Edition, 51(48), pp.12020-12024.
Currier, R.L., Payne, D.C., Staat, M.A., Selvarangan, R., Shirley, S.H., Halasa, N., Boom, J.A., Englund, J.A., Szilagyi, P.G., Harrison, C.J. and Klein, E.J., 2015. Innate susceptibility to norovirus infections influenced by FUT2 genotype in a United States pediatric population. Clinical Infectious Diseases, 60(11), pp.1631-1638.
Uhnoo, I., Olding-Stenkvist, E. and Kreuger, A., 1986. Clinical features of acute gastroenteritis associated with rotavirus, enteric adenoviruses, and bacteria. Archives of Disease in Childhood, 61(8), pp.732-738.
Greenberg, H.B. and Estes, M.K., 2009. Rotaviruses: from pathogenesis to vaccination. Gastroenterology, 136(6), pp.1939-1951.
Brodal, P., 2004. The central nervous system: structure and function. Oxford University Press.
Costantini, T.W., Bansal, V., Peterson, C.Y., Loomis, W.H., Putnam, J.G., Rankin, F., Wolf, P., Eliceiri, B.P., Baird, A. and Coimbra, R., 2010. Efferent vagal nerve stimulation attenuates gut barrier injury after burn: modulation of intestinal occludin expression. The Journal of trauma, 68(6), p.1349.
Michelangeli, F. and Ruiz, M.C., 2003. I, 2. Physiology and pathophysiology of the gut in relation to viral diarrhea. In Perspectives in Medical Virology (Vol. 9, pp. 23-50). Elsevier.
Svensson, L., Desselberger, U., Estes, M. and Greenberg, H. (2016). Viral gastroenteritis: Molecular Epidemiology and Pathogenesis. Scopus.
Das, J.K., Salam, R.A. and Bhutta, Z.A., 2014. Global burden of childhood diarrhea and interventions. Current Opinion in Infectious Diseases, 27(5), pp.451-458.
Payne, D.C., Vinjé, J., Szilagyi, P.G., Edwards, K.M., Staat, M.A., Weinberg, G.A., Hall, C.B., Chappell, J., Bernstein, D.I., Curns, A.T. and Wikswo, M., 2013. Norovirus and medically attended gastroenteritis in US children. New England journal of medicine, 368(12), pp.1121-1130.
Kapikian, A.Z., Wyatt, R.G., Dolin, R., Thornhill, T.S., Kalica, A.R. and Chanock, R.M., 1972. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. Journal of virology, 10(5), pp.1075-1081.
Atmar, R.L. and Estes, M.K., 2001. Diagnosis of noncultivatable gastroenteritis viruses, the human caliciviruses. Clinical microbiology reviews, 14(1), pp.15-37.
van Maarseveen, N.M., Wessels, E., de Brouwer, C.S., Vossen, A.C. and Claas, E.C., 2010. Diagnosis of viral gastroenteritis by simultaneous detection of Adenovirus group F, Astrovirus, Rotavirus group A, Norovirus genogroups I and II, and Sapovirus in two internally controlled multiplex real-time PCR assays. Journal of Clinical Virology, 49(3), pp.205-210.
Dunbar, S.A., 2013. Molecular revolution entering GI diagnostic testing. MLO Med Lab Obs, 45(104), p.8.
Chhabra, P., Gregoricus, N., Weinberg, G.A., Halasa, N., Chappell, J., Hassan, F., Selvarangan, R., Mijatovic-Rustempasic, S., Ward, M.L., Bowen, M. and Payne, D.C., 2017. Comparison of three multiplex gastrointestinal platforms for the detection of gastroenteritis viruses. Journal of Clinical Virology, 95, pp.66-71.
Hurd, S., Patrick, M., Hatch, J., Clogher, P., Wymore, K., Cronquist, A.B., Segler, S., Robinson, T., Hanna, S., Smith, G. and Fitzgerald, C., 2012. Clinical laboratory practices for the isolation and identification of Campylobacter in Foodborne Diseases Active Surveillance Network (FoodNet) sites: baseline information for understanding changes in surveillance data. Clinical infectious diseases, 54(suppl_5), pp.S440-S445.
Reddington, K., Tuite, N., Minogue, E. and Barry, T., 2014. A current overview of commercially available nucleic acid diagnostics approaches to detect and identify human gastroenteritis pathogens. Biomolecular detection and quantification, 1(1), pp.3-7.
Corcoran, M.S., Van Well, G.T.J. and Van Loo, I.H.M., 2014. Diagnosis of viral gastroenteritis in children: interpretation of real-time PCR results and relation to clinical symptoms. European journal of clinical microbiology & infectious diseases, 33(10), pp.1663-1673.
Field, M., 2003. Intestinal ion transport and the pathophysiology of diarrhea. The Journal of clinical investigation, 111(7), pp.931-943.
Apps.who.int. (2018). WHO Drug Information Vol. 16, No. 2, 2002. [online] Available at: http://apps.who.int/medicinedocs/en/d/Js4950e/ [Accessed 21 Oct. 2018].
Ward, R.L., Clemens, J.D., Knowlton, D.R., Rao, M.R., Van Loon, F.P., Huda, N., Ahmed, F., Schiff, G.M. and Sack, D.A., 1992. Evidence that protection against rotavirus diarrhea after natural infection is not dependent on serotype-specific neutralizing antibody. Journal of Infectious Diseases, 166(6), pp.1251-1257.
Feng, N., Lawton, J.A., Gilbert, J., Kuklin, N., Vo, P., Prasad, B.V. and Greenberg, H.B., 2002. Inhibition of rotavirus replication by a non-neutralizing, rotavirus VP6–specific IgA mAb. The Journal of clinical investigation, 109(9), pp.1203-1213.
Corthésy, B., Benureau, Y., Perrier, C., Fourgeux, C., Parez, N., Greenberg, H. and Schwartz-Cornil, I., 2006. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. Journal of virology, 80(21), pp.10692-10699.
Aiyegbo, M.S., Sapparapu, G., Spiller, B.W., Eli, I.M., Williams, D.R., Kim, R., Lee, D.E., Liu, T., Li, S., Woods Jr, V.L. and Nannemann, D.P., 2013. Human rotavirus VP6-specific antibodies mediate intracellular neutralization by binding to a quaternary structure in the transcriptional pore. PloS one, 8(5), p.e61101.
Guzganu, I.L., 2012. Severe diarrhea in a 4-month-old baby girl with acute gastroenteritis: a case report and review of the literature. Case reports in gastrointestinal medicine, 2012.
Matthijnssens, J., Otto, P.H., Ciarlet, M., Desselberger, U., Van Ranst, M. and Johne, R., 2012. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Archives of virology, 157(6), pp.1177-1182.
Patel, M.M., Pitzer, V., Alonso, W.J., Vera, D., Lopman, B., Tate, J., Viboud, C. and Parashar, U.D., 2013. Global seasonality of rotavirus disease. The Pediatric infectious disease journal, 32(4), p.e134.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Healthcare"
Healthcare is defined as providing medical services in order to maintain or improve health through preventing, diagnosing, or treating diseases, illnesses or injuries.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




