Tissue Engineered Cartilage: Bioreactor Mechanically Guided 3D Mesenchymal Stem Cell Chondrogenesis
Info: 8367 words (33 pages) Dissertation
Published: 17th Feb 2022
Tagged: Biology
Bioreactor Mechanically Guided 3D Mesenchymal Stem Cell Chondrogenesis Using a Biocompatible Novel Thermo-Reversible Methylcellulose-Based Hydrogel
Introduction
Structure and Function:
Cartilage is a tough but flexible tissue composed of water and matrix but it lacks a blood supply, nerves, and lymphatic tissue. It is designed to be smooth and have low friction to allow the bones in a joint to glide over one another and allow for smooth, quick, and painless movement. However, when cartilage becomes damaged, the movements can become painful and limit the range of motion of the joint, thus limiting its potential. Cartilage damage can occur for a number of reasons including improper alignment, excessive weight, excessive activity, overuse, or injury [1,2].
On the micro scale, the composition and organization of cartilage is complex with chondrocytes comprising the cellular component. Chondrocytes vary in morphology that ranges in flat and round topographies. Flat chondrocytes are near the surface with rounder chondrocytes in the deeper zones. Chondrocytes vary in shape and pattern depending on the joint type and have mesenchymal stem cell (MSC) properties [1]. It is for these reasons that Cochis and his team choose to explore mesenchymal stem cells as a substitute for articular cartilage, when it becomes damaged.
Although it has been explored that chondrocytes are similar in structure to MSC’s, the MSC’s still require a scaffold structure to be seeded and grow on. In Cochis’ work, the team seeded MSC’s onto a polyurethane (PU) matrix after being suspended in an 8% methylcellulose (MC) solution to serve as the scaffold [3]. The next obstacle in creating chondrocytes is inducing the MSC’s to become chondrocytes. Without external factors, the MSC’s can become a number of cell types, however, extrinsic factors alone may not be enough to promote chondrocyte growth. Previous studies have shown that mechanical loading in combination with exogenous factors during the proliferation stages has had success in increasing the induction of chondrogenesis [4]. In order to simulate the forces cartilage experience, Cochis and his team designed a unique bioreactor that applies both compressive and shear forces to the MSC-MC- PU seeded scaffold to simulate the microenvironment that cartilage experiences during early stages of development [3].
Pathology:
Cartilage defects account for 0.34%, or 43,809 hospital visits annually and that number is expected to increase with time as the population ages and the average life expectancy increases [5]. One of the most prominent diseases associated with cartilage disease is osteoarthritis (OA). OA is most commonly associated with risk factors such as age, injury, gender, obesity, genetic predisposition and abnormal joint shape [6,7]. OA affects all joints in the body creating symptoms such as articular cartilage degradation, subchondral bone thickening, osteophyte formation, synovial inflammation, and ligament degeneration [1]. Changes are often first seen on joint surfaces, where cartilage experiences the most frequent and greatest forces. A key challenge in treating OA is stopping the cartilage damage progression because chondrocytes do not regenerate. A major cause of cartilage destruction is due to the proteinases released by the synovium, which is the junction between the cartilage and synovial pannus. Previous studies show that the synovium is associated with pain and progression of OA and serves as a potential target for cartilage therapies [1].
Another disease commonly associated with cartilage damage is costochondritis. Costochondritis is the swelling and inflammation of the cartilage surrounding the rib and breastbone. The swelling and pain associated with the disease is often mistaken as a heart attack but has no known cause. Current treatment includes minimizing the pain while allowing the area to heal on its own, taking months or up to a year to heal [9]. It can be a result of strenuous physical over-activity, severe respiratory infections, or extreme impacts, such as the chest striking the steering wheel of the car during an automobile accident. Oftentimes, non-steroidal anti-inflammatory (NSAIDs) drugs are prescribed to lessen the pain. In rare cases, surgical removal of the painful cartilage may be necessary to ease the discomfort [9]. Surgery is often a last resort, as cartilage is not regenerative and once the tissue is removed, it cannot be replaced or grown back.
Hernias can also be a result of cartilage failure. The cartilaginous endplate (CEP) is a thin layer of cartilage located between a disc and vertebral body and functions to transport nutrients from blood vessels to the central disc. Cartilage herniation can occur when an intervertebral body ruptures and the soft portion bulges out and puts pressure onto nearby tissue and nerve, causing intense pain [11]. CEP damage can cause further degeneration as the nutrient pathway diminishes and the contact with blood vessels is further lost. Current treatment includes anti-inflammatory drugs, monitoring, and in some cases, surgery [11].
With all of the previously described diseases, the treatment is limited to oral drug therapy (NSAIDs) and surgical removal of cartilaginous tissue. Chondrocytes are low proliferative in their nature and have poor expansion capacity in vitro, therefore, cartilage tissue repair is limited in self-repair capacity [12]. Chondrocyte growth, in vitro, with surgical implantation would allow for a cartilage repair alternative therapy for those that suffer from long-term cartilage diseases, such as osteoarthritis, costochondritis, and cartilaginous hernias.
Understanding the problem:
One of the biggest challenges for tissue engineers surrounding cartilage repair is the tissue’s limited self-renewal capacity. Current cell based therapies include autologous chondrocyte implantation which extracts the patient’s own cartilage from a healthy area, expands the cells in vitro, and then implants them into the damaged area [3]. The limitation of this method is that it relies on the availability of healthy chondrocytes as well as the ability of them to grow ex vivo. The process can include a low cell yield and is not ideal for all patients. Cochis’ work overcomes this obstacle by seeding a PU scaffold with MSC’s to induce a self-repair mechanism [3].
Other current therapies include the use of progenitor cell populations, such as bone marrow derived stem cells (BMSCs), which can differentiate into cartilage cells. In some cases, BMSC’s have been implanted directly into the damaged site or by use of a collagen scaffold or construct. In these cases, mechanical stimuli are a crucial part of development and maintenance because as the stem cells grow in vitro in their early stages, it is important for them to be exposed to the in vivo conditions for the environment that they will be implanted in. For articular cartilage, the natural, in vivo microenvironment includes both compressive and shear forces. In order to simulate these conditions, bioreactors are used to apply these forces in vitro, while the cells reach full maturity [13,14]. For the focus of this paper, Cochis’ work was selected for examination for their use of mesenchymal stem cells, imbedded into a collagen scaffold to test the optimization of their newly developed bioreactor. The bioreactor applies shear and compressive forces to the scaffold prior to subcutaneous implantation into mice to expose the stem cells to an environment during development that would be similar to their long-term goal.
Bioreactors have several key responsibilities including preconditioning implants prior to surgery, providing an ideal microenvironment for stem cells to grow, and representing an effective tool for studying cellular responses to mechanical stimuli. Previous cartilage studies have used bioreactors to study the compressive force reaction on cartilage [10,15] while others have examined shear force [2,5]. The problem with previous designs is that they do not study both compressive and shear force in a single bioreactor and in some cases; the forces applied interfere with the cell-seeded scaffold to properly absorb nutrients [3]. Cochis’ work is novel in that upon examination of cartilage natural environment, they observed that cartilage is subjected to both shear and compressive force and aimed to design a novel bioreactor that mimicked that environment. Furthermore, they asked the question whether these combined, applied forces affected stem cell fate [3].
Overview of comparable strategies:
Strategies in cartilage repair include non-surgical and surgical treatment. Non-surgically, early osteoarthritis is treated with dietary supplement and drugs in combination with physical therapy. However, there is a need to develop better guidelines to balance the efficacy and safety of these drugs. When non-surgical treatment is no longer effective, surgical treatment is the alternative. The most notable surgical approaches are focal cartilage lesions, osteochondral autografting and autologous chondrocyte transplantation. Significantly, autologous chondrocyte transplantation (ACT) procedures are being adopted in studying cartilage repair. Recent research, which focuses on the use of tissue engineering with biomaterials and stem cell therapy for ACT have shown the most potential [16].
Currently, the use of mesenchymal stem cells for cartilage tissue engineering has become a promising cell source. The ability to isolate and expand cells easily in culture, as well as lineage-specific differentiation makes MSC’s ideal for chondrogenesis [17]. There are many uses of MSCs which have already shown clinical success: microfracture of the subchondral bone, intra-articular injections of allogenic MSCs [18] or percutaneous injection of autologous bone marrow derived MSCs [19]. Although these results show strong potential, the tissue formed still “lacks the mechanical properties and structure of articular cartilage needed to sustain the rigorous loading demand” [19]. Therefore, a more favorable path to functionally repair and restore damaged cartilage is ACT; which uses the tissue engineering approach of integrating MSCs into biomaterials in combination with in vitro manipulations or exogenous stimuli [20].
In terms of biomaterials, various scaffolds has been used in vitro and in vivo such as agarose [21], hydrogels [22], alginate [23], fibrin [24] , hyaluronan [25] and collagen [26]. Overall, poly(a-hydroxyacids)s and Poly(ester-urethane) are most popularly used as synthetic foam structures. Other novel biomaterial approach includes: thermo-responsive smart hydrogels [3] and polysaccharide scaffolds in combination with magnetic cell seeding [27].
Concerning in vitro manipulation, many groups have investigated in the effects of various chemical cues such as growth factors like TGFβ1, TGFβ3, insulin-like growth factor and interleukins [28,29,30]. The TGFβ family and bone morphogenetic proteins have shown to be the most important inducers of chondrogenesis [31]. Another novel in-vitro manipulation is the use scaffold-free tissue-engineered constructs under low oxygen tension conditions [32].
When examining exogenous factors, there are various physical stimuli, including hydrostatic pressure, tension, compression and shear stress that exist in the natural joint loading environment [33]. Therefore, various mechanical stimulation mimicking the natural stimuli, have been developed such as sliding contact loading, physiologic deformational loading, delayed compressive loading, and multi-axial loading [34, 35, 36, 37]. Some groups have created bioreactors that can apply compression: strain [38], shear [39], cyclic [35]. Moreover, many studies have indicated that mechanical forces are able to modulate the fate of the MSCs when correctly applied [31]. Therefore, increasingly complex and novel bioreactor systems are being developed such as a shear and dynamic compression bioreactor [4], ultrasonic bioreactor [40] and biaxial bioreactor [41].
Although, there has been considerable success using MSC in cartilage tissue engineering constructs, there still lacks a successful method to recreate a functional articular cartilage that could simulate the original tissue [20] The complexity of this issue suggests that there should be joint approach from multiple disciplines (biology, biomechanics, biomaterials science) and high-throughput analysis approaching the clinical goal of treating articular cartilage diseases [38, 42]. Therefore, a successful tissue engineered model for chondrogenesis should evaluate mechanical stimulation, chemical stimulation and include a biomaterial [17, 43].
Experimental Design
Overall Goal:
Current cartilage therapeutic research is a lengthy and expensive process and can lead to total joint replacement, if it fails. The first step involved in autologous chondrocyte transplantation involves harvesting chondrocytes from an uninjured area of the patient using arthroscopy technology. Next the chondrocytes are expanded in vitro for 2 to 3 weeks, and then reinjected into the damaged site. This procedure has promising results in early clinical evaluation but is limited by the chondrocyte’s expansion ability and maintenance in vitro [14].
As a result of the limitations of chondrocyte proliferation and growth, newly developed cell therapies have explored new cell populations, such as bone marrow derived mesenchymal stem cells (BMSC’s). BMSC’s, as well as mesenchymal stem cells (MSC’s), have the ability to differentiate into cartilage cells and have been previously used for repair of articular cartilage through the use of scaffold embedding and injection [14, 15]. However, one challenge with using MSC’s is that they require a scaffold to support their growth and proliferation. Previous studies have investigated the use of polyethylene glycol (PEG), enriched with RGD peptides, as a supporting matrix material and have looked at the interaction between PEG and chondrocytes while under compressive force. [10] It was found that compressive loading did not significantly affect the chondrocyte activity, however, chondrocyte gene expression showed an upregulation under mechanical loading conditions, when compared to those in static conditions. This emphasizes the importance of mechanical loading, prior to implantation, to assimilate the chondrocytes to their newly formed environment.
In Cochis’ work, their team selected a polyurethane (PU) to seed the MSC’s on and added a methylcellulose (MC) solution to give the hydrogel thermo-reversible mechanical properties in response to physiological changes. This combined hydrogel of MSC’s, MC, and PU is evaluated in vitro under compressive and shear stress which also served as the necessary mechanical stimulation prior to implantation. In addition to the in vitro analysis and preparation, the hydrogel is tested in vivo for biocompatibility. From this work, the feasibility for a MSC hydrogel is determined to examine possibility for clinical use.
Experimental Design and application to a physiological system (in vitro/in vivo):
In vitro:
A bioreactor is necessary to have a representation of physiological processes in the human body and allows for implantable tissues and other devices to be tested by carrying out a biological reaction in vitro. In the case of Cochis et al. [3], their bioreactor allows for loading conditions to be simulated prior to in vivo testing for cartilage tissues.
For this study, the in vitro testing includes characterizing the mechanical properties of the hydrogel. MSC’s are seeded onto a PU scaffold and the scaffold is secured in place by a Polyether ether ketone (PEEK) ring, as seen in Figure 1.
The purpose of this experiment is to apply physio-mechanical forces to form the MSC’s for tissue regeneration. On a large-scale application, this step is necessary to prepare the MSC’s for surgical use and allow for a study tool to examine the cellular response to these forces.
Previous studies by Campbell et al. [38] have examined compressive force reaction on MSC’s. Their team applied compressive force at a strain of 15%, frequency of 1 Hz, and compared the results with and without the presence of 10 ng/mL TGFβ3 for 8 days. The results showed significant changes to the level of mRNA expression for chondrocytes in the presence of TGFβ3, implying that the bioreactor used in combination with TGFβ3 promotes chondrocyte proliferation and formation [38].
Furthermore, Huang’s team examined the possible use of agarose gel as a supportive scaffold for chondrogenesis of human-bone marrow mesenchymal stem cells (hBM-MSC’s). His team compared cell-seeding densities of 3, 6, and 9 × 106 cells/ml grown in medium containing TGFβ3.
Real time- polymerase chain reaction (RT-PCR) was performed to analyze chondrogenesis of the hBM-MSC’s and found that the cultures in the agarose gels were higher than the initial cell seeding densities and contained more cartilage-specific gene expression markers [21]. This work implies that agarose is an adequate scaffold biomaterial for growing hBM-MSC’s to induce chondrogenesis invitro. For the purposes of cartilage, compressive and shear forces should be examined for it to be considered representative of human, cartilage tissue.
Previous studies have also examined shear forces, such as the work by Chang et al [8]. Their bioreactor design consisted of two, glass, cylindrical chambers, each with four branch tubes (medium flow & outflow, O2 ventilation, surplus), as seen in Figure 2. The magnetic bar stirring provides the mechanical, shear stress, as would be in cartilage tissue. However the shear forces examined by Chang et. al. are not realistic to a natural environment because cartilage experiences both shear and compressive stresses. It is because of these reasons that the bioreactor created by Cochis’ team is more representative of the physio-mechanical forces in the body. The hip ball provides compressive stress when applied in a downward motion and while the ball rests on top of the scaffold, it rotates providing shear stress [3].
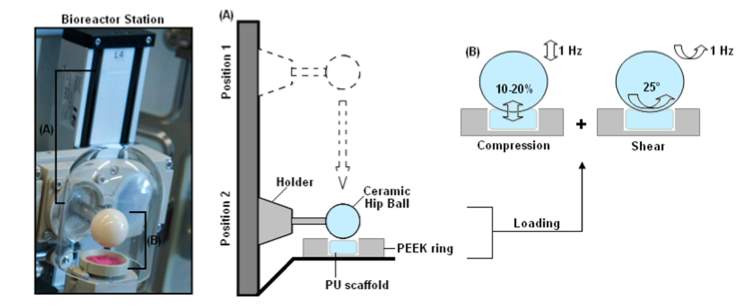
Figure 1: Bioreactor Design by Cochis et al. [3]
A) Mobile hip ball applying a vertical, compressive force to a PU scaffold, held in place with a PEEK ring, controlled remotely by external software
B) Hip ball applying a compressive force of 1 Hz, as well as a shear force of a 25° rotation
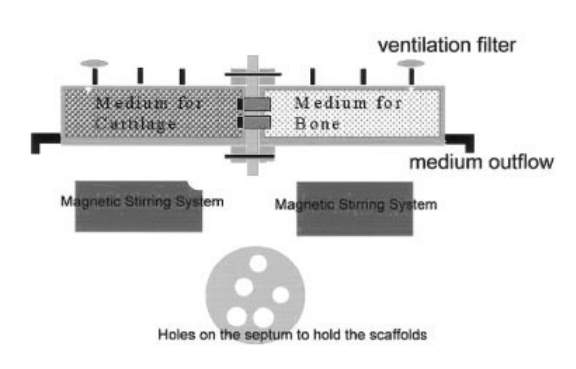
Figure 2: Bioreactor Design by Chang et al. [8]
The dual chamber bioreactor contains two, glass, cylindrical tubes with four branch tubes for medium inflow & outflow, O2 ventilation, and surplus use. The two, main chambers are separated with a porous, rubber septum while the magnetic stirring bar provides the mechanical stimulation.
In vivo:
It is essential for all implantable, injectable, and ingestible materials to be tested in vivo to ensure a minimal immune reaction and to measure biocompatibility. In the case of Cochis’ work, MSC survival and differentiation is dependent on the cell’s ability to adhere and grow/spread onto a substrate, more specifically in this case, the hydrogel. Recent biomedical applications have given rise to the new field of smart polymers. Smart polymers are high performance polymers that are able to change physiological, chemical, or mechanical properties in response to their environment, such as a change in pH or temperature. The polymers can be synthesized as hydrogels, combined into composites, or electrospun into microfibers [13]. Their versatility allows them to be manipulated to be biodegradable, biocompatible, and optimized for specific use. Furthermore, their conductive nature allows the cells cultured upon them to be electrically stimulated and easily influenced by drugs [13].
Smart polymers, or more specifically, smart hydrogels, experience rapid, reversible changes to their microstructure when changing from a hydrophilic to hydrophobic environment. At high temperatures, the polymer-polymer interactions are dominant and water becomes a poor solvent, creating a gel- like structure. It is for these reasons that Cochis’ team chose to seed MSC’s onto a methylcellulose (MC) hydrogel, which would allow for thermo-reversible behavior in both hydrophilic and hydrophobic environments. Previous work has demonstrated the dependence of viscosity on temperature at well-defined shear rates, shown in Figure 3. Newtonian behavior is demonstrated at temperatures lower than 30°C and becomes non-newtonian with increasing shear rates. The threshold at which the critical temperature changes from a newtonian to a non-newtonian fluid depends on the molecular weight of the MC hydrogel [13]. As hydrogels transition between states of matter, it makes them widely applicable for both injections as well as scaffolds [3].
For the purposes of this work, an MC-derived hydrogel is investigated as a 3D injectable matrix guided by BMSC chondrogenesis on a polyurethane (PU) scaffold. Molecular weight of the MC is determined by rheological characterization at the optimal body temperature of 37°C. Following fluid dynamic evaluation, BMSC’s are seeded with the hydrogel onto the PU scaffold and are mechanically stimulated, using the previously described bioreactor from Figure 1. At the time of 21 days, the composites are collected and evaluated for chondrogenic activity using RT-PCR, histology, and immunofluorescent assays to assess the effectiveness of the mechanical stimulation on stem cell differentiation.
Following evaluation, cylindrical hydrogel samples are subcutaneously implanted into 6-8 week old wild type mice. To determine cellular immune response, mice are sacrificed at either 3 or 6 weeks, and spleens are dissected and assayed to determine lymphocyte proliferation. Cells are suspended in Alpha Minimal Essential Medium, supplemented with 10% FBS and 1% antibiotics with a cell density of 2.5 × 106 cells/mL. Next, 500 μl of cell suspension are pipetted onto a 24 well plate containing 100 μl of hydrogel or PBS as negative control. Mitogen ConA (5 mg/mL) serves as the positive control to confirm lymphocyte activity. Then, after 48 hours, cell viability is determined by MTT assay and cellular response by Stimulation Index. These results are compared to the density of cells in the presence on the hydrogel and mitogen ConA.
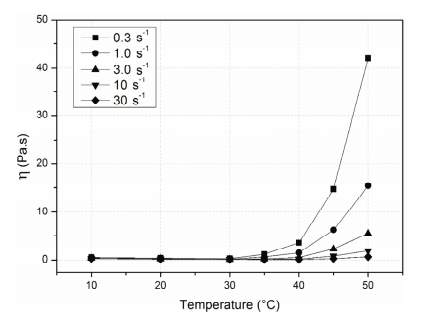
Figure 3: Critical Temperature Effect on Viscosity of MC at Varying Shear Rates [8]
The MC hydrogels exhibit newtonian behavior for temperatures less than 30°C. The critical temperature at which the hydrogels switch from newtonian to non-newtonian behavior is dependent on molecular weight.
Overview of their findings (summarize):
A mesenchymal stem cell seeded hydrogel has high potential for both in vitro and in vivo applications due to its thermo-responsive properties and low inflammatory response. The purpose of this study is to evaluate an MC hydrogel as a 3-dimensional matrix to promote and sustain chondrocyte growth from human MSC’s. In order to do this, Cochis’ team needed to create novel bioreactor that would apply both shear and compressive force. Their approach includes the use of a ceramic hip ball, PEEK ring, and disc shaped PU scaffold, all under the control of external, computer software. Once prepared, the scaffold goes into the bioreactor where it experiences shear and compressive forces. It is then implanted subcutaneously into mice and tested by immunostaining for chondrogenesis. In order to measure the immune reaction of the mice, the spleens are weighed and tested for lymphocyte activity and red & white blood cell counts are measured and compared between the two time points of 3 and 6 weeks.
The results suggest that the MC hydrogel’s thermo-responsive behavior and biocompatibility would make it a good scaffold for chondrocyte delivery. The results for biocompatibility show no sign of immune response, with similar spleen weights between the 3 and 6-week time points. Additionally, red and white blood cell counts were identical between the control and experimental groups. Concerning the effectiveness of the bioreactor, GAG is higher in the mechanically stimulated MSC’s than the non-stimulated cells, indicating differentiation for MSC’s. This is further supported by the results of the histological analysis that show cellular retention on the PU scaffold in the presence of a collagen matrix. This indicates that the MC-hydrogel would be suitable for carrying and promoting growth of MSC’s for chondrogenesis.
Discussion
Current challenges and the study’s success:
In the past two decades, there has been consistent success in tissue engineering for autologous chondrocyte implantation. However, there are still challenges that need to be overcome. Articular cartilage shows a low intrinsic capacity for repair after injuries due to the intrinsic avascular connective tissue nature and limited cellularity of the tissue [44]. Autologous chondrocyte implantation is hindered by the in-vitro limited expansion of chondrocytes and arduous maintenance [45]. Specifically, common problems in tissue engineering include cell leakage, poor cell survival/differentiation and poor integration [16]. Articular cartilage is a highly organized structure composed of 3 zones with specific properties and functions [42]. Therefore, biomaterials need to have nonlinear, non-homogeneous, viscoelastic properties mimicking the native cartilage as well as being biodegradable, biocompatible and bioactive [16]. An additional challenge is that the static cultures do not represent the natural environment of the native cartilage tissue [46]. In fact, various mechanical forces are closely related to the cartilage microenvironment such as shear, tension, compression and hydrostatic pressure [33]. Therefore, there exists a need for an established model of bioreactor tailoring for cartilage proliferation that extensively mimics the native environment.
Cochis et al. have successfully addressed these challenges in their study. Concerning cell sourcing, this study uses readily available MSC’s that have the capacity for quick expansion and can easily differentiate into cells from various mesenchyme-derived tissues such as fat, bone, and tendon. Regarding biomaterials and in-vitro manipulation, Cochis’ group took a novel approach of focusing on biomaterials by using the MC-derived smart hydrogels in combination with a porous PU scaffold. These biodegradable hydrogels promote cell adhesion and have the ability to change physicochemical/mechanical properties when the specific external pH/temperature changes. Specifically, MC based hydrogels have a thermo-reversible behavior. Potentially, these smart hydrogels could be used as a 3D injectable matrix for cell delivery and various tissue applications. MC-based hydrogels are superior to natural polymers like alginate, agarose and silk because they are highly customized with specific mechanical properties. They also have been shown to induce and support chondrogenesis. MC-based hydrogels are superior to synthetic hydrogels because they do not require other biomolecules (such as dexamethasone and TGFβ) to promote cell adhesion and proliferation. Therefore, no TGFβ was used in this study. Without depending on active biomolecules’ absorption and lifespan, the efficacy of a material can also be evaluated and quantified objectively. Considering bioreactors, Cochis’ group custom designed a bioreactor to apply shear force superimposed upon dynamic compression. This model mimics the kinematic motion of an articulating joint of the native system using a model of a rolling ceramic ball. In previous studies, this model of bioreactor has shown to lead to significant increases in chondrogenic gene expression [4]. Overall, the combination of a smart MC-based hydrogel and a dual purpose bioreactor; this study provides an effective model to further investigate chondrogenesis as well as contribute to various cell delivery and tissue engineering applications [3].
Comparable Work
Many studies have focused on the use of cartilage tissue engineering with the use of mesenchymal stem cells to produce functional replacement tissue. These studies fall into two, main categories: biomaterials or biochemical stimulation. Very few studies have designed a chondrocyte-specific mechanical stimulation model and even fewer studies utilize mesenchymal stem cells to test them. Although, all of these studies have all-around multidisciplinary approach, the focus here will be on mechanical stimulation model and bioreactors.
A Medline search was performed for all published literature using an original search of (mesenchymal stem or MSC) and (chondrogenesis) or (chondrocyte) or (chondrocytes) or (cartilage) and (bioreactor) or (mechanical stimuli) or (physical stimuli), which resulted in over 150 references. Of these references, the most current studies (from 2007to 2017) were selected and ones involving bioreactors specifically compatible to cartilage or chondrogenesis were examined. The review below emphasizes the mechanical stimuli aspects of the work presented previously by A. Cochis et. al “Bioreactor mechanically guided 3D mesenchymal stem cell chondrogenesis using a biocompatible novel thermoreversible methylcellulose-based hydrogel” [3] with the work of Subramanian et al. in “Growth factor and ultrasound-assisted bioreactor synergism for human mesenchymal stem cell chondrogenesis” [40] and the work of Nathalie Luciani et al. in “Successful chondrogenesis within scaffolds, using magnetic stem cell confinement and bioreactor maturation [27]. In summary, Cochis’ group uses MC smart hydrogels in combination with a porous PU scaffold and the bioreactor characterized by a moving and rolling ceramic ball. Subramanian’s group uses a combination of ultrasound at 5 MHz and transforming growth factor β3 with polyurethane-urea-based macroporous 3D scaffolds and ultrasound-assisted bioreactor. Luciani’s group used magnetic condensation of MSC and polysaccharide scaffold and the TisXell bioreactor.
In Cochis’ study, the bioreactor is custom designed to mimic the kinematic motion of an articulating joint. This bioreactor can simultaneously apply cyclic axial compression with surface shear to a developing cell scaffold construct. The mechanical stimulus that applied force to the PU scaffold was a pin-on-ball bioreactor system. The hip ball is a 32-mm ceramic ball pressed onto the PU scaffold (Figure 1). Interface shear force is created by oscillation of the ceramic ball along the y-axis. Simultaneously, compression is generated throughout the cylindrical axis of the PU scaffold (Figure 4) [3,4].
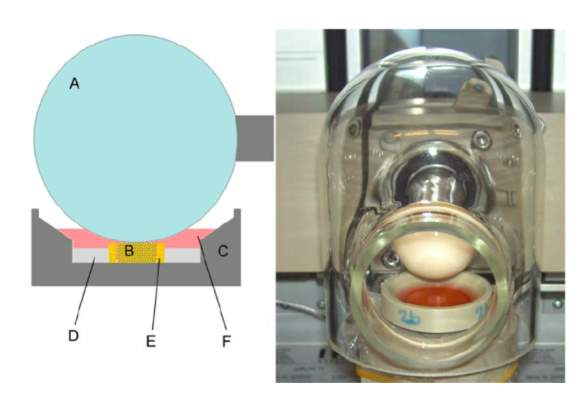
Figure 4: Bioreactor by Schatti et al. [4]
Left: Diagram of cross section of scaffold with its holder
Right: Real time image of bioreactor
In a study by Thakurta et al., a bioreactor is composed of an incubator and a transducer array. The incubator, “Forma Model 3033 Steri-Cult incubator”, is utilized to control temperature, humidity & CO2 and provide a sterile environment. An insert for nine plates is custom design so that the media can be changed and a transducer array can excite the plates systematically. The transducer array includes six ultrasonic transducers (Olympus V309, 5MHz). The transducers are positioned within the fluid filled cavity for water coupling (between transducers and the bottom of the plates). USB function generator (Tektronix AG-3012B) is used to control the amplitude and frequency of excitation. Stages can be computed with great flexibility through the linear stages of motion: x-y and z. The x-y stages move the transducer to the right position under the plates and the z stage raises and lowers the transducers during each ultrasound cycle (Figure 5) [40].
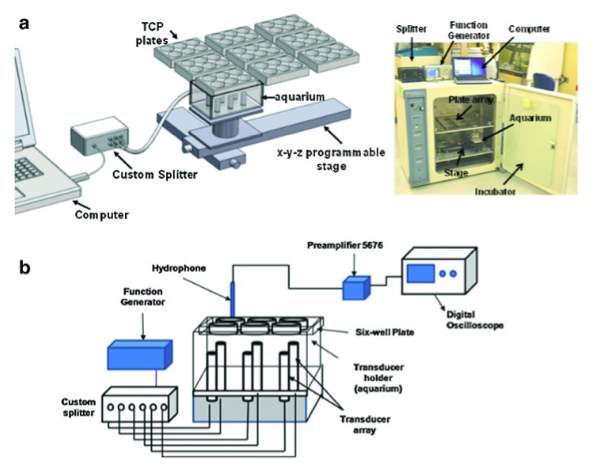
Figure 5: Bioreactor by Subramanian et al. [40]
A. Programmable stage allow movement in the x, y, and z directions; the plate holds the six well plates above a transducer tray
B. Organization of the data acquisition model
In Luciani’s study, the group used TisXell by Quinxell Techonologies, a commercially available bioreactor (Figure 6) [27]. In this patented design, there are three modes: biaxial, single axis and swing modes that allow for arm rotation ranging from 1 to 12 rpm as well as direction of rotation. There is continuous perfusion providing flow rates from 3 to 500 ml/min. An oxygenator column unit to facilitates gaseous exchange. The Tisxell bioreactor is used by industrial laboratories for cartilage regeneration.
Figure 6: TisXell by Quinxell Techonologies [27]
A commercially available bioreactor
Although each of these bioreactors are different in purpose, the bioreactor designs can still be comparable based on various mechanical parameters that have been shown to exit in the natural join loading environment such as: osmotic loading, hydrostatic pressure, electro kinetic phenomena, compression and shear forces [17]. In terms of mechanical stimuli, the pin-on-ball bioreactor shows the closest resemblance to articular joint with the combination of simultaneous compression and shear stress. The TisXell system and the ultrasonic bioreactor, however, do not provide such mechanical stress [46]. Furthermore, the ultrasonic stimulation has been shown to influence cell growth, however comprehensive studies with different parameters have not been performed [47]. Although both studies of the TisXell System and the ultrasonic bioreactor have shown successful chondrogenesis, other important variables exist that make it difficult to assess these bioreactors’ effectiveness; specifically, the use of growth factors. TisXell is a great commercially available bioreactor that provide various applications for institutions, hospitals, pharmaceutical biomaterials, and stem cell expansion, however, the unique and complex biology of chondrogenesis may require more cartilage-specific features in this system for an optimal apparatus.
Importance
Cochis et al. has successfully induced MSC chondrogenesis. In vitro, the MC hydrogel has shown the reversible thermo-responsive properties. Different temperatures change the hydrogels’ mechanical properties in accordance with previous literature. It takes the hydrogel one day of submersion in PBS to degrade which, suggests a potential for long application times incubated in culture media. In vivo, no inflammatory reaction was observed and H/E staining showed no differences in the controls versus the mice implanted with hydrogels. No significant difference was noted in both the 3 and 6 weeks for the control compared to the experimental group in terms of spleen weight and lymphocyte reactivity. Red and white cell counts between the controls and experimental group did not vary significantly between both time points. COL2, SOX 9 and ACAN in the hydrogels group (markers for chondrogenesis) had a 64-fold increase and were significantly higher than the control group. COL 10 and COL1 (markers for hypertrophy) did increase, however, it is insignificant when compared to the increase of COL2, SOX9 and ACAN. In conclusion, this study used MC hydrogel in combination with mechanical stimulation to promote chondrogenesis in MSCs. Biocompatibility is evaluated for the MC hydrogels, as a 3D matrix to support the mechanical induction of chondrogenesis. MC hydrogel is demonstrated to be a suitable vehicle for cell delivery. This study presents a customized chondrogenesis-specific bioreactor successfully mimicking the natural joint. This study also presents a comprehensive model of a chondrogenic study, taking into account the union of biomaterials and bioreactors, in vitro and in vivo. In the current progress of tissue engineering for autologous chondrocyte implantation, this model streamlines the overall results and makes the data meaningful and comparable.
References
1. Goldring M. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Therapeutic Advances In Musculoskeletal Disease [serial online]. January 1, 2012;4(4):269-285.
2. Eustice, Carol. “10 Interesting Facts About Cartilage.” Verywell. Osteoarthritis, 22 Aug. 2016.
3. Cochis A, Rimondini L, Alini M, et al. Bioreactor mechanically guided 3D mesenchymal stem cell chondrogenesis using a biocompatible novel thermo-reversible methylcellulose-based hydrogel. Scientific Reports [serial online]. March 23, 2017;7.
4. Schätti, O. et al. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur Cell Mater. 22, 214–25 (2011).
5. “Statistics about Cartilage Disorders.” Statistics about Cartilage Disorders – RightDiagnosis.com. Right Diagnosis, 13 Aug. 2015.
6. Blagojevic, M., Jinks, C., Jeffery, A. and Jordan, K.P. (2010) Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 18: 24–33.
7. Felson, D.T., Lawrence, R.C., Dieppe, P.A., Hirsch, R., Helmick, C.G., Jordan, J.M. et al. (2000) Osteoarthritis: new insights. Part 1: The Disease and its risk factors. Ann Intern Med 133: 635–646.
8. Chang C, Lin F, Lin C, Chou C, Liu H. Cartilage tissue engineering on the surface of a novel gelatin-calcium- phosphate biphasic scaffold in a double-chamber bioreactor. Journal Of Biomedical Materials Research – Part B Applied Biomaterials [serial online]. November 15, 2004;71(2):313-321
9. Costochondritis: not a heart attack but it feels like one. Harvard Women’s Health Watch [serial online]. March 2003;10(7):6-7.
10. Nicodemus, G. D. & Bryant, S. J. The role of hydrogel structure and dynamic loading on chondrocyte gene expression and matrix formation. J Biomech. 41, 1528–36 (2008).
11. Joe E, Lee J, Yeom J, et al. Herniation of cartilaginous endplates in the lumbar spine: MRI findings. American Journal Of Roentgenology [serial online]. May 1, 2015;204(5):1075-1081.
12. Appelman, T. P., Mizrahi, J., Elisseeff, J. H. & Seliktar, D. The differential effect of scaffold composition and architecture on chondrocyte response to mechanical stimulation. Biomaterials. 30, 518–25 (2009).
13. Pauline L. N, Frédéric P, Joana L. M. S, Maria Eugênia R. D, Miguel D. N, Marguerite R. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers, Vol 7, Iss 5, Pp 777-803 (2015) [serial online]. 2015;(5):777.
14. Brittberg M. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. Biology Digest [serial online]. October 6, 1994.
15. Shen, Y., Fu, Y., Wang, J., Li, G., Zhang, X., Xu, Y. Z. et al. Biomaterial and mesenchymal stem cell for articular cartilage reconstruction. Curr Stem Cell Res Ther. 9, 254–67 (2014).
16. Rai, Vikrant, Matthew F. Dilisio, Nicholas E. Dietz, and Devendra K. Agrawal. “Recent Strategies in Cartilage Repair: A Systemic Review of the Scaffold Development and Tissue Engineering.” Journal of Biomedical Materials Research Part A (2017): n. pag. Web.
17. Tan, Andrea R., and Clark T. Hung. “Concise Review: Mesenchymal Stem Cells for Functional Cartilage Tissue Engineering: Taking Cues from Chondrocyte-Based Constructs.” STEM CELLS Translational Medicine 6.4 (2017): 1295-303.
18. Vangsness, C. Thomas, Jack Farr, Joel Boyd, David T. Dellaero, C. Randal Mills, and Michelle Leroux-Williams. “Adult Human Mesenchymal Stem Cells Delivered via Intra-Articular Injection to the Knee Following Partial Medial Meniscectomy.” The Journal of Bone and Joint Surgery-American Volume 96.2 (2014): 90-98. Web.
19. Centeno, C. J., D. Busse, J. Kisiday, C. Keohan, M. Freeman, and D. Karli. “Increased Knee Cartilage Volume in Degenerative Joint Disease Using Percutaneously Implanted, Autologous Mesenchymal Stem Cells.” Pain Physician11.3 (2007): 343-53. Web.
20. Bernhard, Jonathan C., and Gordana Vunjak-Novakovic. “Should We Use Cells, Biomaterials, or Tissue Engineering for Cartilage Regeneration?” Stem Cell Research & Therapy. BioMed Central, 18 Apr. 2016. Web. 02 May 2017.
21. Huang C, Reuben P, D’Ippolito G, Schiller P, Cheung H. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anatomical Record Part A-Discoveries In Molecular Cellular And Evolutionary Biology [serial online]. n.d.;278A(1):428-436.
22. Kisiday JD, Kopesky PW, Evans CH, Grodzinsky AJ, McIlwraith CW, Frisbie DD. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008;26:322–331. doi: 10.1002/jor.20508.
23. Diduch DR, Jordan LC, Mierisch CM, Balian G. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy. 2000;16:571–577. doi: 10.1053/jars.2000.4827.
24. Li Z, Kupcsik L, Yao S, Alini M, Stoddart MJ. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites. Tissue Eng Part A. 2009;15:1729–1737. doi: 10.1089/ten.tea.2008.0247.
25. Angele P, Schumann D, Angele M, Kinner B, Englert C, Hente R, Fuchtmeier B, Nerlich M, Neumann C, Kujat R. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology. 2004;41:335–346.
26. Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. doi: 10.1002/bit.20828.
27. Luciani, Nathalie, Vicard Du, Florence Gazeau, Alain Richert, Didier Letourneur, Catherine Le Visage, and Claire Wilhelm. “Successful Chondrogenesis within Scaffolds, Using Magnetic Stem Cell Confinement and Bioreactor Maturation.” Acta Biomaterialia 37 (2016): 101-10.
28. Awad H, Halvorsen Y-DC, Gimble JM et al. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose- derived stromal cells. Tiss Eng 2003;9:1301– 1312.
29. Byers BA, Mauck RL, Chiang I et al. Temporal exposure of TGF-B3 under serum-free conditions enhances biomechanical and bio- chemical maturation of tissue-engineered cartilage. Trans Orthop Res Soc 2006;31:43.
30. Choukair, Daniela, Ulrike Hügel, Anja Sander, Lorenz Uhlmann, and Burkhard Tönshoff. “Inhibition of IGF-I-related Intracellular Signaling Pathways by Proinflammatory Cytokines in Growth Plate Chondrocytes.” Nature News. Nature Publishing Group, n.d. Web. 02 May 2017.
31. Tan, Andrea R., and Clark T. Hung. “Concise Review: Mesenchymal Stem Cells for Functional Cartilage Tissue Engineering: Taking Cues from Chondrocyte-Based Construcs.” STEM CELLS Translational Medicine – Issue – Wiley Online Library. N.p., n.d. Web. 02 May 2017.
32. Yasui, Yukihiko, Ryota Chijimatsu, David A. Hart, Kota Koizumi, Norihiko Sugita, Kazunori Shimomura, Akira Myoui, Hideki Yoshikawa, and Norimasa Nakamura. “Preparation of Scaffold-Free Tissue-Engineered Constructs Derived from Human Synovial Mesenchymal Stem Cells Under Low Oxygen Tension Enhances Their Chondrogenic Differentiation Capacity.” Tissue Engineering Part A 22.5-6 (2016): 490-500. Web.
33. Grad, Sibylle, David Eglin, Mauro Alini, and Martin J. Stoddart. “Physical Stimulation of Chondrogenic Cells In Vitro: A Review.” Clinical Orthopaedics and Related Research® 469.10 (2011): 2764-772. Web.
34. Lima EG, Bian L, Ng KW et al. The bene- ficial effect of delayed compressive loading on tissue-engineered cartilage constructs cul- tured with TGF-beta3. Osteoarthr Cartil 2007; 15:1025–1033.
35. Hung CT, Mauck RL, Wang CCB et al. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng 2004; 32:35
36. Mauck, Robert L., Michael A. Soltz, Christopher C. B. Wang, Dennis D. Wong, Pen-Hsiu Grace Chao, Wilmot B. Valhmu, Clark T. Hung, and Gerard A. Ateshian. “Functional Tissue Engineering of Articular Cartilage Through Dynamic Loading of Chondrocyte-Seeded Agarose Gels.” Journal of Biomechanical Engineering. American Society of Mechanical Engineers, 01 June 2000. Web. 02 May 2017.
37. Bian L, Fong JV, Lima EG et al. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A 2010;16:1781–1790
38. Campbell J, Lee D, Bader D. Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology [serial online]. 2006;43(3-4):455-470.
39. Shahin, Kifah, and Pauline M. Doran. “Shear and Compression Bioreactor for Cartilage Synthesis.” Cartilage Tissue Engineering Methods in Molecular Biology (2015): 221-33. Web.
40. Subramanian, Anuradha, Joseph A. Turner, Gaurav Budhiraja, Sanjukta Guha Thakurta, Nicholas P. Whitney, and Sai Siddhartha Nudurupati. “Ultrasonic Bioreactor as a Platform for Studying Cellular Response.” Tissue Engineering Part C: Methods 19.3 (2013): 244-55.
41. Quinxell. N.p., n.d.
42. Johnstone, Brian, Mauro Alini, Magali Cucchiarini, George R. Dodge, David Eglin, Farshid Guilak, Henning Madry, Alvaro Mata, Robert L. Mauck, Carlos E. Semino, and Martin J. Stoddart. “Tissue Engineering for Articular Cartilage Repair – The State of the Art.” European Cells and Materials. Swiss Society for Biomaterials, 01 Jan. 1970.
43. Balint R, Cassidy N, Cartmell S. Review: Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomaterialia [serial online]. June 1, 2014;10:2341-2353.
44. Tew, Simon, Samantha Redman, Alvin Kwan, Elizabeth Walker, Ilyas Khan, Gary Dowthwaite, Brian Thomson, and Charles W. Archer. “Differences in Repair Responses Between Immature and Mature Cartilage.” Clinical Orthopaedics and Related Research 391 (2001): n. pag.
45. Huselstein, C., Y. Li, and X. He. “Mesenchymal Stem Cells for Cartilage Engineering.” Bio-medical Materials and Engineering3rd ser. 22.1 (2012): 69-80. Print.
46. Ravichandran, Akhilandeshwari, Yuchun Liu, and Swee-Hin Teoh. “Review: Bioreactor Design towards Generation of Relevant Engineered Tissues: Focus on Clinical Translation.” Journal of Tissue Engineering and Regenerative Medicine (2017): n. pag.
47. Thakurta, Sanjukta Guha, Gaurav Budhiraja, and Anuradha Subramanian. “Growth Factor and Ultrasound-assisted Bioreactor Synergism for Human Mesenchymal Stem Cell Chondrogenesis.” Journal of Tissue Engineering 6 (2015): 204173141456652.
Team Member Breakdown
Introduction:
Structure/Function: Michelle
Pathology: Michelle
Experimental design contribution: Michelle
Overview of strategies: Tien
Experimental Design:
Goal: Michelle
Design and application: Michelle
Overview: Michelle
Discussion:
Author Success: Tien
Comparable work: Tien
Importance: Tien
References: Both
Editing: Both
Power point slides:
Tien: 7-12
Michelle: 1-6
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biology"
Biology is the scientific study of the natural processes of living organisms or life in all its forms. including origin, growth, reproduction, structure, and behaviour and encompasses numerous fields such as botany, zoology, mycology, and microbiology.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




