Dissertation on Thrombotic Thrombocytopenic Purpura
Info: 9374 words (37 pages) Dissertation
Published: 17th Nov 2021
Abstract
Thrombotic Thrombocytopenic Purpura (TTP) is a rare blood disorder characterized by clotting in small blood vessels of the body (thromboses), resulting in a low platelet count. In its full-blown form, the disease consists of the pentad of microangiopathic haemolytic anaemia, thrombocytopenic purpura, neurologic abnormalities, fever, and acute renal damage/failure.
Assays have been developed to measure von Willebrand factor cleaving enzyme (ADAMTS-13) activity. ADAMTS-13 activity can be low, and inhibitors to its activity can often be demonstrated in patients with TTP. These are confirmatory tests, as the results are not returned in time to make a prompt diagnosis. There is debate over whether the ADAMTS-13 activity assay can help in the management of patients with TTP. It does not appear to predict who will respond to plasma exchange. Studies have shown that the multiple domains of ADAMTS-13 are frequently targeted by anti-ADAMTS-13 immunoglobulins (inhibitors) in patients with acquired (idiopathic) TTP. Immunosuppression could work by reducing this inhibitor (Starke R et al, 2006).
In UCLH we currently have an assay for testing ADAMS-13 activity which enables us to quantify ADAMTS-13 levels and aids in diagnosing TTP patients. Going one further and testing for ADAMS13 antibodies/inhibitors will allow us to further investigate why the ADMATS-13 activities are low, questioning whether its congenital and or idiopathic auto-immune.
This procedure is the first to be researched at UCLH laboratories, and so this assay will further our knowledge and skills in regards to the affects and diagnosis in TTP patients which may alter the clinical outcomes and treatment protocols for patients at UCLH.
This study used the Technozym ADAMTS13 inhibitor assay. Doing this investigation, I compared two different buffers: di-sodium Hydrogen orthophosphate dodecahydrate and Citric Acid anhydrous, also verifying whether antibodies affect the assay by using two groups of Lupus Positive and Syphilis patients. I also looked into how this assay is affected by patients who have had pre- and post plasma transfusion.
I found that 38% showed to exhibit positive IgG values for Lupus and syphilis patients indicating that autoantibodies present in these two states are causing TTP. From these set of results Citric Acid anhydrous provided me with false negative results, which concluded that this buffer was not stable for this assay. Plasma transfused patients, 68% provided negative IgG values after plasma transfusion and 33% still remained elevated post transfusion. This suggesting that, ADAMTS13 analysis may still provide understanding to the disease and the concept of ADAMTS13 binding IgG is helpful in understanding weather it is congenital and or idiopathic auto-immune.
Click to expand Table of Contents
CONTENTS
Introduction……………………………………………………………………..4
Von Willebrand Factor (vWF)………………………………………………4
Fig. 1. VWF monomer and multimers structure………………………….5
ADAMTS13……………………………………………………………………5
Structure of ADAMTS13………………………………………………….5-6
Figure 2. Domain organisation of ADAMTS13…………………………..6
Function of ADAMTS13…………………………………………………….6
ADAMTS13 – VWF interaction……………………………………………..6
Figure 3. Interaction of VWF and ADAMTS13…………………………..7
Pathophysiology of TTP…………………………………………………..7-8
Figure 4. Aggregation of platelets………………………………………8
Thrombotic Thrombocytopenic Purpura (TTP)……………………….8-9
Discovering TTP………………………………………………………….9-10
Treatment of TTP…………………………………………………………..10
Method and material…………………………………………………….11
Specimen requirements…………………………………………………11
Interpretation of results…………………………………………………11
Results…………………………………………………………………….12
Statistical analysis………………………………………………………12
Table 1. Summary of results…………………………………………..12
Figure 5. Representative reference curve…………………………….13
Figure 6. Graph – Normal population…………………………………13
Figure 7. Graph – Lupus Positive comparison of buffers……………14
Figure 8. Graph – Syphilis positive comparison of buffers…………14
Figure 9. Graph – Pre-& Post Plasma transfusion…………………..15
Table 2. T-Test – Pre-& Post ……………………………………………16
Table 3. T-Test – Lupus positive……………………………………….16
Table 4. T-Test – Syphilis Positive…………………………………..17
Discussion ……………………………………………………..…….17-19
Conclusion………………………………………………………………..19
Further work……………………………………………………………..19
References …………………………………………………………..20-21
Appendix …………………………………………………………….22-27
ABBREVATIONS
| ADAMSTS13 | A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 |
| ANA | Antinuclear Antibodies |
| BSA | Bovine serum albumin |
| FVIII | Factor VIII |
| HRP | Horseradish peroxidase |
| MAHA | Microangiopathic haemolytic anaemia |
| NHS | National Health Service |
| OPD | Ortho phenylene diamine dihydrochloride |
| PBS | Phosphate buffered saline |
| Rh-ADAMTS13 | Recombinant human ADAMTS13 |
| SLE | Systemic lupus erythematosus |
| SOP | Standard Operating Procedure |
| TTP | Thrombotic thrombocytopenic purpura |
| VWF | Von Willebrand factor |
| VWFCP | Von Willebrand factor-cleaving protease |
| WHO | World Health Organisation |
Introduction
Thrombocytes also known as platelets, are components in the blood. They are crucial to normal blood clotting. They do this by clumping together and clotting blood vessel injuries.
The role of platelets is to maintain haemostasis – which is the process of stopping bleeding at the site of disturbed endothelium. Platelets gather at the site and form a plug in three steps. The first step is adhesion, where the platelets attach to the element outside the interrupted endothelium. (Mari R Thomas et al 2015)
Next is activation, where the shape of the platelets change, which allow receptors to become active and secrete chemical messengers and finally aggregation, where the platelets connect through receptor bridges”.
The formation of the platelet plug (primary haemostasis) is related to the activation of the coagulation cascade, following fibrin deposition and linking (secondary haemostasis).
Thrombocytopenia is a term used for low platelets concentration. It is the result of either decreased production or an increase in destruction. An increase in platelet count is termed thrombocytosis. This is either congenital, reactive or due to unregulated production.
Normal platelets can react to an abnormality on the vessel wall rather than to haemorrhage. This leads to inappropriate platelet adhesion/activation and thrombosis – Clot formation within an intact vessel. This type of thrombosis will act differently to that of a normal clot.
Excessive and spontaneous bleeding occurs due to platelet disorders. It could be caused due to a number of reasons: deficient numbers of platelets, dysfunctional platelets or excessive number of platelets. The excessive numbers cause a von Willebrand factor (vWF) deficiency due to sequestration. (Mari R Thomas et al 2015)
Von Willebrand Factor (vWF)
A blood glycoprotein – Von Willebrand factor (vWF) is involved in the process of haemostasis. It is deficient or defective in von Willebrand disease. It is also involved in other diseases. These include: Thrombotic thrombocytopenic purpura (TTP), Heyde’s syndrome, and could be involved in haemolytic-uremic syndrome.
VWF is an essential part of platelet – subendothelium adhesion and platelet-platelet cohesion and aggregation in vessels with elevated shear stress. Adhesion is promoted by the interaction of a region of the A1 domain of vWF with platelet GpIb. The understanding is that high stress levels will activate the A1 domain of the collagen-bound VWF by stretching vWF multimers into a filamentous form.
Aggregation of platelets within the growing hemostatic plug is promoted by the interaction with a second receptor on platelets, GPIIb-IIIa, which after activation binds to VWF and fibrinogen, recruiting more platelets into a stable plug.
4.2 Fig. 1. VWF monomer and multimers structure. Available from http://www.practical-haemostasis.com/Factor [Accessed 16th March 2018]
The main role of VWF is binding to other proteins, the main one being Factor VIII (FVIII). It is also very important in platelet adhesion to wound sites. As vWF is not an enzyme, catalytic activity does not happen.
As FVIII binds to VWF during circulation, however t is inactive. FVIII rapidly degrades when it is not attached to vWF. Through thrombin, FVIII is released from vWF. Due to damage within the blood vessels, the endothelial cells are exposed. This is when vWF binds to collagen. The endothelium releases vWF, which causes the formation of links between the platelets glycoprotein Ib/IX/V and the collagen fibrils (Shangbin Yang et al 2011).
VWF binds to platelet gpIb. It does this when it forms a complex with gpIX and gpV. This interaction will occur all the time but is more effective under high stress. vWF will bind to other platelet receptors when they are activated.
ADAMTS13 (A Disintegrin And Metalloprotease with ThromboSpondin 1 Repeats)
ADAMSTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) is an enzyme that biological breakdown vWF. It is also known as a metalloproteinase. It cleaves vWF between the tyrosine at position 842 and methionine at position 843 at the A2 domain. The multimers are broken down into smaller units, which are then destroyed by other peptidases. ADAMSTS13 is also given the name von Willebrand factor-cleaving protease (vWFCP).
Structure of ADAMTS13
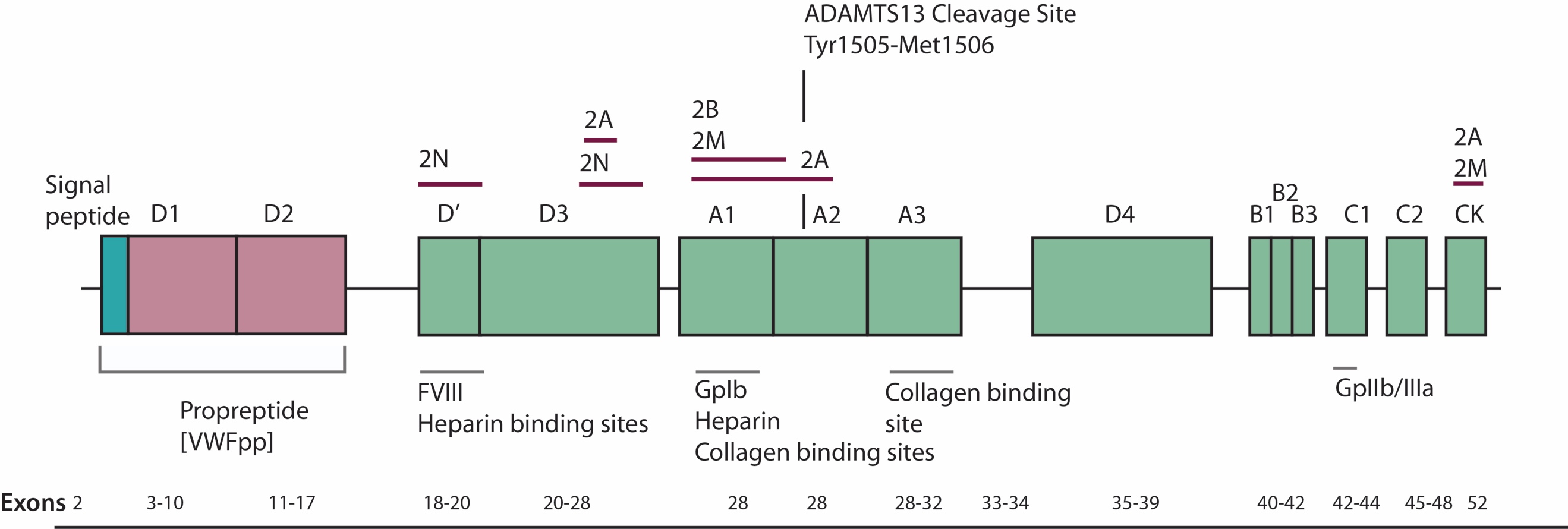
ADAMTS13 contains 1427 amino acids residues. Within the structure of ADAMTS13 there is a N terminal and a C terminal. Between these consists of a propeptide region, metalloprotease domains (MP), disintedrin like domain (Dis), thrombospondin type 1 motif (TSP1), cysteine-rich domain (Cys), spacer domain, TSPI repeats which include seven of these (TSP2-8) and finally CUB domains of which are two. Figure 2 shows the domain establishment of ADAMTS13.
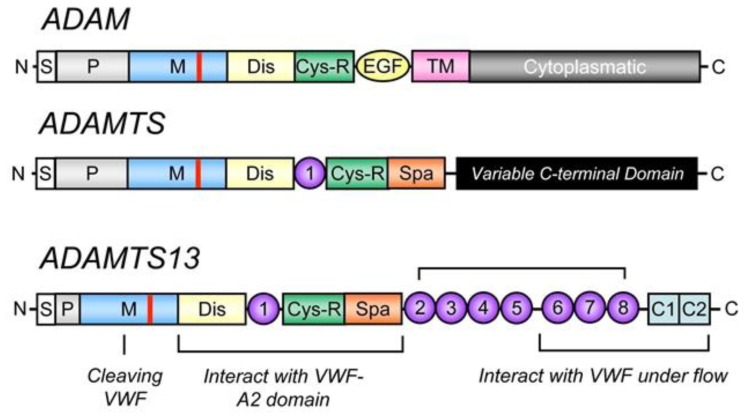
4.5. Figure 2. Domain organisation of ADAMTS13 (Mari Rebaca Thomas, 2016)
Function of ADAMTS13
The functions of ADAMTS13, is it functions as a VWF cleaving protease. It works by cleaving peptide bonds: Tyr-Met which are located in the central VWF A2 domain (Jacob et al, 2006). In order to control VWF multimers proteolysis is important. In order studies, it has been shown for ADAMTS13 to decrease vascular inflammation (Fred et al, 2009).
ADAMTS13 – VWF interaction
The interaction between VWF and ADAMTS13 binding, implicates an involvement and specific set of interactions that are reliant on the validation of the substrate – VWF. ADAMTS13 doesn’t have a zymogen state, hence the enzyme is always active when secreted. Compared to other protease in haemostasis, ADAMTS13 doesn’t have a known inhibitor and so the inhibitor is prompted according to the VWF changes when introduced to shear stress (Rens et al, 2015).
Acknowledgement of VWF by ADAMTS13 comprises of the interaction between the distinct domains of two of the proteins.
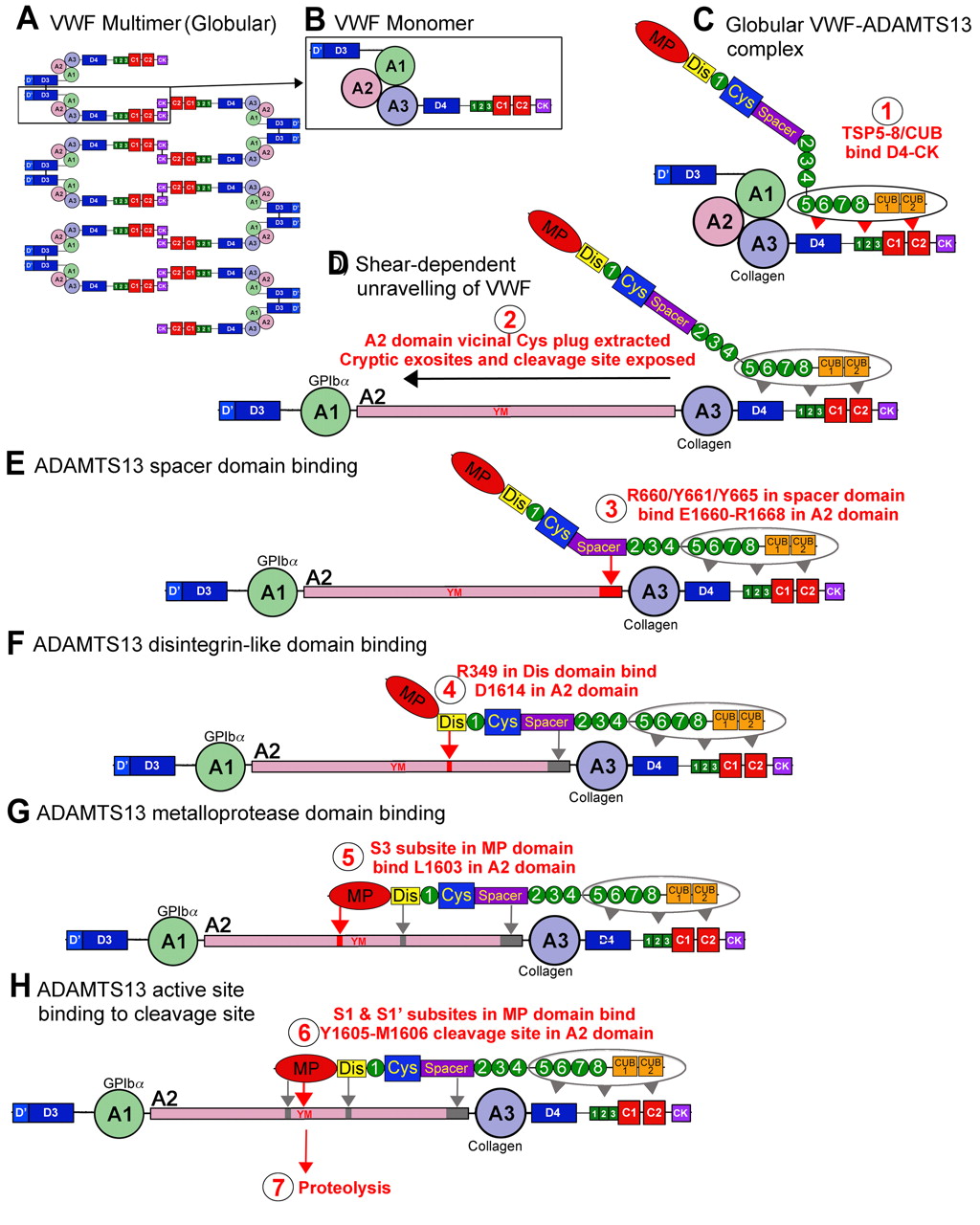
4.8 Figure 3. Interaction between ADAMTS13 and VWF (Crawley et al, 2011)
Pathophysiology of TTP
To understand the pathophysiology of TTP, we must first understand platelet function, vWF and the proteolytic enzyme ADAMTS13 when the endothelium is injured – to which all is described above.
The role of the platelets is to clot in order to close damaged blood vessels. The activation and number of platelets at the damaged site is all reliant on vWF which is synthesised by endothelial cells. These cells are located near the damaged site and respond by producing vWF which is secreted in the form of large multimeric chains. (Shangbin Yang et al 2011)
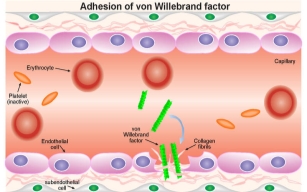
4.10 Fig. 4 https://www.immunopaedia.org.za [Accessed 16th March 2018]
The fragments of vWF attach to the visible subendothelial collagen. GP1b a cell surface receptor which the platelets express. This allows them to adhere and detect to vWF bound to collagen fibrils. Platelets will further express cell surface receptors, such as GPIIb/IIIa – which mediate platelet binding to other platelets through fibrinogen intermediates.
These factors contribute to the increase of platelets to the site of endothelium damage and platelet aggregation to form a seal in the breached endothelium. The platelets also initiate the blood clotting cascade that generates a meshwork of insoluble fibrin.
Thrombotic Thrombocytopenic Purpura (TTP)
A rare disorder of the blood coagulation system, known as Thrombotic Thrombocytopenic Purpura (TTP) causes low platelet counts due to a widespread of clots in the small blood vessels throughout the body. Kidneys, heart, brain and the nervous system could be damaged due to these small clots, also known as thrombosis.
There are five symptoms that are associated with TTP. These are: neurological symptoms, renal dysfunction, pyrexia and microangiopathic haemolytic anaemia with thrombocytopenia. In the microcirculation, the formation of platelet microthrombi is the cause for most of the symptoms that are seen in the TTP, and these result in organ ischaemia.
TTP causes vulnerability to the endothelia of the kidneys, heart, pancreas, brain, adrenal glands and the spleen. (Shangbin Yang et al 2011)
Organs less affected are the lungs, liver, gallbladder, retina, ovaries, gastrointestinal tract, skeletal muscles, uterus and testes.
TTP occurs due to the derived plasma proteolytic enzyme called ADAMTS13, this cleaves the large multimeric chains of VWF into smaller fragments. However, because of an enzyme, this is depleted.
Through molecular mimicry, autoantibodies can be produced. This is followed by a humoral response, which is due to an infection and or disruption or tolerance to self-antigens. This has been observed in autoimmune diseases like SLE or during HIV infection. It was researched that during HIV infection there is an interruption of CD4 and T cell regulation and an increased risk of other infections.
When ADAMTS13 is absent, the large chains of vWF multimers increase in the circulation and bind to exposed subendothelial collagen fibrils, which start the recruitment of high numbers of platelets to the sites of the endothelium damage.
Because, of the large number and size of these chains, platelets are increases and activated. These result in the following: Platelet depletion or thrombocytopenia, microangiopathic haemolytic anaemia (MAHA) which is caused via the impedance of red cells through small blood vessels causing the cells to shear.
Discovering TTP
TTP had been known to be one of the microangiopathic haemolytic anaemia in 1982. It was characterised in its familial form by the amount of unusually large von Willebrand factor multimers (ULVWF) that was found in the plasma.
It was discovered in 1994, that vWF attached itself between a tyrosine at positon 1605 and a methionine at 1606. This was cleaved by a plasma metalloprotease enzyme when exposed to levels of high stress.
In 1996, the enzyme was further researched. The development of platelet microthrombi in the blood vessels was found to be the congenital deficiency of vWF cleaving protease. They also reported that IgG antibodies that were detected against the same enzyme that caused TTP in a majority of non-familial causes. (Clive Gray et al 2014)
Upshaw Schulman Syndrome, discovered that the recurring familial form of TTP was due to the deficiency of ADAMTS13. Within this time, it had already been discovered that TTP occurred in the autoimmune form which was due to its response to plasmapheresis and the classification of IgG inhibitors. There have been epitopes on the surface of ADAMTS13 that have been discovered and recognised as target of inhibitory antibodies.
The fatality rate was at 90% before the effective treatment of plasma exchange came into the picture. With plasma exchange, this has dropped to 10% at six months.
TTP commonly results from antibodies. These antibodies activate the immune system to prevent the ADAMSTS13 enzyme. Following plasma exchange, if the patient was to relapse or the recurrence of the disease was to occur, the following agents can be used to suppress the immune system: glucocorticoids, rituximab, cyclophosphamide, vincristine or cyclosporine.
Patients would not receive a platelet transfusion unless, they have a life-threatening bleed and since the transfused platelets would also quickly be consumed by thrombosis formation, leading to a minimal increase in circulating platelets.
Treatment of TTP
TTP can be diagnosed and treated by using exchange therapy or plasma infusion. This provides the missing enzyme ADAMTS13 and refurbishes proteolytic cleavage of the large multimeric chains of vWF into smaller fragments, therefore avoiding unnecessary platelet aggregation into small blood vessels.
Plasma exchange therapy works by contributing to the removal or reduction of the autoantibodies to ADAMTS13 by diluting them. (Clive Gray et al 2014)
Plasma infusion or exchange therapy may not always deliver a long-term benefit. This originates from where the inhibition of ADAMTS13 is due to the persistence of autoantibody producing B lymphocytes, that are not removed by plasma treatment. In these situations, the treatment with rituximab is considered. It works by CD20 binding monoclonal antibody that selectively binds to the B lymphocytes and facilitates their destruction. Removal of the source of the autoantibody, results in restoration of significant ADAMTS13 activity.
Assays have been developed to measure von Willebrand factor cleaving enzyme (ADAMTS-13) activity. ADAMTS-13 activity can be low, and inhibitors to its activity can often be demonstrated in patients with TTP. These are confirmatory tests, as the results are not returned in time to make a prompt diagnosis. There is debate over whether the ADAMTS-13 activity assay can help in the management of patients with TTP. It does not appear to predict who will respond to plasma exchange. Studies have shown that the multiple domains of ADAMTS-13 are frequently targeted by anti-ADAMTS-13 immunoglobulins (inhibitors) in patients with acquired (idiopathic) TTP. Immunosuppression could work by reducing this inhibitor.
In UCLH we currently have an assay for testing ADAMS-13 activity which enables us to quantify ADAMTS-13 levels and aids in diagnosing TTP patients. Going one further and testing for ADAMS13 antibodies/inhibitors will allow us to further investigate why the ADMATS-13 activities are low, questioning whether its congenital and or idiopathic auto-immune.
This procedure will be the first to be researched at UCLH laboratories, and so this assay will further our knowledge and skills in regards to the affects and diagnosis in TTP patients which may alter the clinical outcomes and treatment protocols for patients at UCLH.
The analysis of ADAMS13-binding IgG will help us with the diagnosis and management of TTP. This assay is not an assay that we currently do, so this will help us with our clinical applications.
We hope that this ADAMTS13 analysis may provide valuable insight to the disease status during the course of therapy. Analysis of ADAMTS13-binding IgG will help us in the diagnosis and management of TTP.
Methods and materials
A total of 100 samples were tested for this study. I obtained various sample types that included samples from patients with various antibodies and normal patients. From these, patients had plasma transfusions and or acute TTP. Two different buffers were used: di-sodium Hydrogen orthophosphate dodecahydrate and Citric Acid anhydrous.
Microwells are coated with full-length recombinant ADAMTS13. During the incubation period, diluted plasma samples are added, the autoantibodies existing to ADAMTS13 present in the sample will fix to the protein coated in the wells. After a washing step, a rabbit anti-human IgG antibody that is labelled with horseradish peroxidase (HRP) is added to the microwells. This binds to IgG type ADAMTS13 autoantibodes that have been bound to the coated microwells. Another washing step is done. Substrate (Orthophenylene Diamine, OPD) is added and the reaction with the HRP produces a yellow coloured solution.
The reaction is stopped by the adding of sulphuric acid, which turns the solution colour orange. The absorbance is measured at 490nm. The value from the standard curve concludes the level of ADAMTS13 autoantibodies in dilutes plasma sample. ADAMTS13 autoantibody levels are expressed in arbitrary units as a percentage of the reactivity of an index, strong positive reference plasma. Refer to appendix 1 for more details about the method used for this study.
Specimen Requirements
This assay is validated for the use of citrated human plasma. IgG anti-ADAMTS13 may also be detected in serum, however levels may vary due to the potential impact of platelets bound ADAMTS13 and sequestration of autoantibodies.
The results will be reported as percentages which is relative to the standard curve.
Interpretation of results
I established a reference range which I obtained an upper limit of 6.1% calculated as the 95th percentile of 49 normal healthy donors. A previous normal range established using a lower purity form oh rh –ADAMTS13 in 46 normal subjects yielded a cut off value of 4.2% (Starke et al, 2006).
Results
See appendix 3 for full results
Statistical analysis
The statistical analysis for this study was carried out using Microsoft excel. Results on continuous measurements were presented as Mean ± SD (Min-Max) and results on categorical measurements were presented in number (%). An independent t-test has been carried out to determine if there was a significant difference between the turnaround times of the tests.
| Group | Number of cases | Platelet count median (range) x109/l | ADAMTS13 inhibitor positive % |
| Normal Patients | 50 | 210 (138-581) | 0 |
| TTP plasma | 12 | 34 (9-38) | 73 |
| Positive antibodies | 25 | 26 (3-128) | 14 |
| Acute TTP | 13 | 11 (2-140) | 93 |
6.2 Table 1. Summary of the positive ADAMTS13 inhibitor including the platelet count.
This table shows the 100-case study tested with the di-sodium Hydrogen orthophosphate dodecahydrate. The 13 cases for the acute TTP showed to have 93% positive for ADAMTS13 inhibitor, followed by patients transfused with plasma with 73% and the positive antibodies with 14% (25 out of 100).
A standard curve of the antibody assay is shown in figure 1. The assay used a standard curve for the dilutions of the Index Reference Plasma using a linear curve Fit (y=mx+c) and read of the values for each QC and test plasma.
The upper limit of normal was 6.1%. Results above this range or above the upper most standard curve point required repeating with 1:2 dilutions with PBS: BSA buffer.
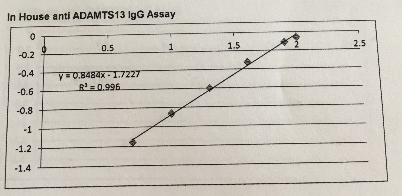
6.3 Figure 5. A representative reference curve for determination of ADAMTS13-binding IgG levels.
The equation for this curve: y = mx+c hence x= (y-c)/m
y = median OD m=0.848376 c= -1.72275 y=0.8484x-1.7227: R2 = 0.996
The IgG concentration were reported in percentages using this calibration curve with the absorbance at 450nm.
6.4 Figure 6. Graph representing the normal population of patients using the IgG inhibitor assay
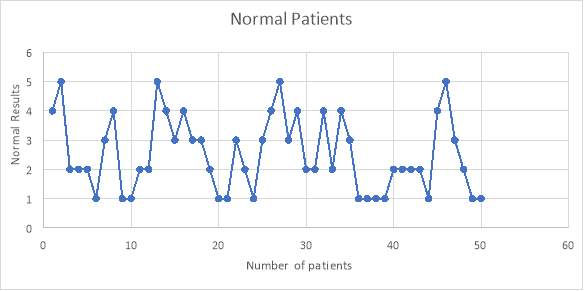
From the 50 normal patients that were tested, I found that none of the patients had any IgG antibodies that affected the testing of the assay. This was shown with the results being less than 6% as negative.
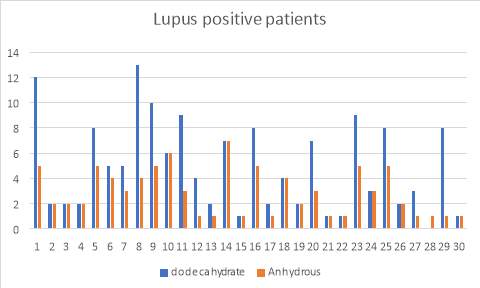
6.5 Figure 7. Graph representing the Lupus positive patients using the IgG inhibitor assay comparing between dodecahydrate and anhydrous buffer
6.6 Figure 8. Graph representing the Syphilis positive patients using the IgG inhibitor assay comparing between dodecahydrate and anhydrous buffer
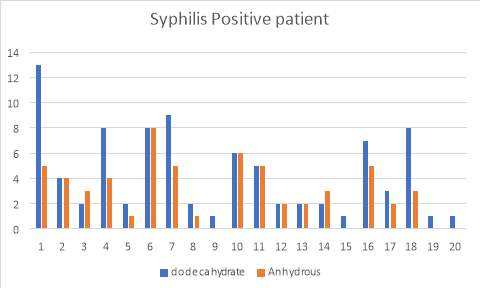
Figure 3 and 4 show the results for the 50 patients that were positive for lupus as well as Syphilis. Within the 50, I also had patients that had acute TTP and had plasma transfusion. The samples were run and compared with the two buffers and as you can see, the di-sodium Hydrogen orthophosphate dodecahydrate buffer provided more accurate results than the Citric Acid anhydrous buffer which provided me with false negative results.
I found that the patients with recent plasma exchange or transfusion falsely normalised ADAMTS13 antibody levels. (full results shown in the appendix).
38% of the patients (19 of 50 cases) showed to present positive IgG binding values compared to the 62% (31 of 50 cases) which didn’t. Furthermore, I investigated further into the patients and found that they all had low ADAMTS13 activity levels. 24% (12 of the 19 cases) of the Lupus positive patients exhibited high IgG levels, whereas 14% (7 of 19) of the syphilis patients showed high IgG levels.
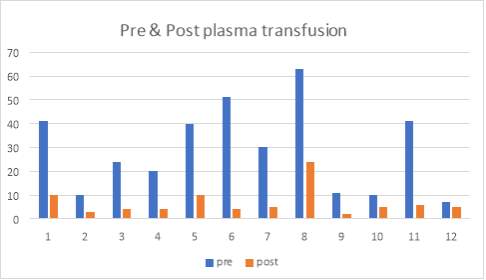
I also did further analysis of the 50 cases and determined whether or not they had plasma transfusion and so figure 5 I have provided data that relieved the results of the IgG antibody levels of 12 cases with post and pre-plasma transfusion.
67% (8 of 12 cases) showed that there was no presence of ADAMTS13 IgG antibody after the patient had plasma transfusion, however 33% (4 of 12 cases) showed that after the plasma transfusion, the values still remained elevated.
6.7 Figure 9. Graph showing the pre-and post-plasma transfusion results of IgG antibody levels of the 12 patients
Null hypothesis
The null hypothesis states that there is no significant difference between the buffers and the post and pre -plasma transfusion.
6.8 Table 2. t-Test: Paired Two Sample for Means for the pre- and post -plasma transfusion for patients
| t-Test: Paired Two Sample for Means | ||
| Pre | Post | |
| Mean | 29 | 6.833333333 |
| Variance | 335.0909091 | 35.24242424 |
| Hypothesized Mean Difference | 0 | The null hypothesis can be rejected. There is a significant difference between the Pre-and Post-plasma transfusion. P value < 0.01 are considered significant. The values for pre-and post-transfusion results are statistically significant (p=<0.01). |
| DF | 11 | |
| t Stat | 5.242784749 | |
| P(T<=t) two-tail | 0.000275636 | |
| t Critical two-tail | 2.20098516 | |
6.9 Table 3. t-Test: Paired Two Sample for Means for the Lupus positive patients compared to the two buffers
| t-Test: Paired Two Sample for Means | ||
| Lupus dodecahydrate | Lupus Anhydrous | |
| Mean | 4.9 | 2.9 |
| Variance | 12.98965517 | 3.334482759 |
| Hypothesized Mean Difference | 0 | The null hypothesis can be rejected. There is a significant difference between the two buffers for Lupus positive patients. P value < 0.01 are considered significant. The values for the two buffers are statistically significant (p=<0.01). |
| DF | 29 | |
| t Stat | 4.171330723 | |
| P(T<=t) two-tail | 0.000250776 | |
| t Critical two-tail | 2.045229642 | |
6.10. Table 4. t-Test: Paired Two Sample for Means for the syphilis positive patients compared to the two buffers
| t-Test: Paired Two Sample for Means | ||
| Syphilis dodecahydrate | Syphilis Anhydrous | |
| Mean | 4.35 | 2.95 |
| Variance | 11.92368421 | 5.207894737 |
| Hypothesized Mean Difference | 0 | The null hypothesis can be rejected. There is a significant difference between the two buffers for Syphilis positive patients. P value < 0.01 are considered significant. The values for the two buffers are statistically significant (p=<0.01). |
| DF | 19 | |
| t Stat | 2.80295203 | |
| P(T<=t) two-tail | 0.011350433 | |
| t Critical two-tail | 2.093024054 | |
From the results above it can be concluded that the two buffers when compared to the Lupus and syphilis samples are statistically significant p = <0.01. All statistical analysis was conducted using Microsoft Excel.
Discussion
The special coagulation department at University College London Hospital (UCLH) currently has an ongoing test for ADAMTS13-IgG antibody assay. The test uses the Elisa method which detects the human autoantibodies (IgG) in serum or plasma against the ADAMTS13. Currently at UCLH we have an assay for testing ADAMTS13 activity which enables us to quantify ADAMTS13 levels and aids in diagnosing TTP patients. In this investigation, I went one further and investigated ADAMTS13 antibodies/inhibitor which will provide a better understanding why the activity is low and questioning whether it is congenital or idiopathic auto-immune.
I initially performed the assay using two different buffers: Dodecahydrate and anhydrous buffer. The anhydrous buffer provided false negative results which showed that this buffer wasn’t able to perform accurately for this assay. There are many reasons as to way it provided false negative results. As in the name ‘anhydrous’ no water was added to this buffer. Buffers require solubility and hence should be very soluble in water (Ferrari et al 2009). Solubility will stop the buffer was gathering in the cell membrane, non-polar compartments and vesicles. The buffer included citrate and phosphate which are high in ions. This may have adjusted the salts in the compound which may have influenced the stability of the buffer (Han-Mou et al, 2006). Comparing this to the second buffer I used: dodecahydrate, this buffer was used for all the assays and provided accurate results.
Tween 20, was used as a blocking reagent in the Elisa assay. As this is a detergent, that is non-ionic we used it as an agent in the assay due to the stability and groundwork of constant oil in water suspensions. This was used in the assay for the pre-extraction of the membranes to eliminate any peripheral proteins. So, I found that the Tween 20 buffer, had sufficient stability and worked well in obtaining accurate results for this assay.
Conditions and the use the correct buffer all do matter in this assay. It was very important that I obtained that.
In this study, I also investigated the existence of ADAMTS13 binding IgG is in line with the fact that autoantibodies of ADAMTS13 cause absence of the enzyme which means that VWF will not be broken down leading to TTP.
Lupus and Syphilis were two of the autoantibodies that I investigated further to determine if the antibodies would affect the assay. I looked into 50 TTP patients. From this I found that 38% showed to have high IgG levels. With the antibody present, these patients all had low ADAMTS13 activity which means that the protein doesn’t cleave VWF hence leading to TTP. I further investigated these patients and I found that it was the antibody that was affecting the breaking down process of VWF. Along with the antibody blocking ADAMTS13 I also found that some patients with TTP was due to genetic defects which could also lead to TTP. With these patients, the Elisa test wouldn’t detect the values and so further testing would be required.
The cases that I chose, some patients had more than the one autoantibody, which wasn’t detected using the Elisa method. I looked into this and found that the cases needed to be followed up using the Bethsa method. This method involved incubation and looking at a different way of detecting these antibodies.
There are pathological reasons as to why some of the results were unexpected: the assay holds limitations in that some of the samples had high concentrations of anti ADAMTS13 autoantibodies which resulted in positive or borderline results.
Regardless of how well the assay is refined, pathologically they will be variations. This is based on the patient’s background, genetic mutations and if they have developed different antibodies (Technoclone, 2017).
With this, the results show that this assay is specific and sensitive in the recognition of antibodies of ADAMTS13.
The percentage of the patients after receiving plasma transfusion was 67%, this was due the fact that the plasma transfusion had lowered the free ADAMTS13 binding antibody levels. The difference of the pre-and post-plasma transfusion did have some inferences. As the results show, I had obtained borderline or low levels of ADAMTS13. However, this didn’t exclude the diagnosis of TTP (Robert S. et al, 2006). The reduction of ADAMTS13-binding IgG detected after the plasma transfusion is consistent with other observations made: in that, TTP patients with low levels of inhibitor did improve after the transfusion.
The acutely high levels of ADAMTS13 antibody’s may not be detected by the Elisa method, presumably due to the ‘hook effect’ or because of the presence of another antibody subtype and or non-specific binding from other IgG antibodies, I found had also falsely raised results. So, in this instance, repeating with fresh samples after a few days of the first presentation tended to show more clinically consistent results. For this reason, I found that the clinician was frequently requesting repeats to verify the initial results (Mudde C et al, 2009).
Conclusion
In conclusion, the study demonstrates that ADAMTS13 analysis provides a valuable insight to the disease status during the course of therapy. It has helped us in the diagnosis and management of TTP. I have found that this assay makes it possible to differentiate between congenital (gene polymorphisms) and acquired (autoantibodies) TTP when coupled to an activity assay. It’s also managed to control efficacy of plasma exchange therapy (S. Ferrai et al, 2009).
Assays have been developed to measure von Willebrand factor cleaving enzyme (ADAMTS-13) activity. ADAMTS-13 activity can be low, and inhibitors to its activity can often be demonstrated in patients with TTP. These are confirmatory tests, as the results are not returned in time to make a prompt diagnosis. There is debate over whether the ADAMTS-13 activity assay can help in the management of patients with TTP. It does not appear to predict who will respond to plasma exchange. Studies have shown that the multiple domains of ADAMTS-13 are frequently targeted by anti-ADAMTS-13 immunoglobulins (inhibitors) in patients with acquired (idiopathic) TTP. Immunosuppression could work by reducing this inhibitor, which has been demonstrated in this investigation.
At UCLH this assay has been implemented and I found that this assay is very specific and sensitive in the detection of autoantibodies. So, there shouldn’t be any interference from other antibodies.
Future work
Future work will involve the identification of this assay using citrate and plasma samples to determine if these affect the IgG antibodies and determine which IgG subclass the ADAMTS13 antibodies are affecting.
References
Andre P, Denis CV, Ware J, Saffaripour S, Hynes RO, Ruggeri ZM, Wagner DD. Platelets adhere to and translocate on von Willebrand factor presented by endothelium in stimulated veins. Blood 2000; 96: 3322–8.
Ben Addi A, Cammarata D, Conley PB, Boeynaems JM, Robaye B. Role of the P2Y12 receptor in the modulation of murine dendritic cell function by ADP. J Immunol 2010; 185: 5900–6.
Bennett CL, Connors JM, Carwile JM, Moake JL, Bell WR, Taran- tolo SR, McCarthy LJ, Sarode R, Hatfield AJ, Feldman MD, Davidson CJ, Tsai HM. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med 2000; 342: 1773–7.
deGrootR, BardhanA,RamroopN,LaneDA,CrawleyJT.Essential role of the disintegrin-like domain in ADAMTS13 function. Blood 2009; 113: 5609–16.
Ferrari S, Scheiflinger F, Rieger M, Mudde G, Wolf M, Coppo P, Girma JP, Azoulay E, Brun-Buisson C, Fakhouri F, Mira JP, Oksenhendler E, Poullin P, Rondeau E, Schleinitz N, Schlemmer B, Teboul JL, Vanhille P, Vernant JP, Meyer D, et al. Prognostic value of anti-ADAMTS 13 antibody features (Ig isotype, titer, and inhibitory effect) in a cohort of 35 adult French patients undergoing a first epi- sode of thrombotic microangiopathy with undetectable ADAMTS 13 activity. Blood 2007; 109: 2815–22.
Fontana S, Hovinga JA, Studt JD, Alberio L, Lammle B, Taleghani BM. Plasma therapy in thrombotic thrombocytopenic purpura: review of the literature and the Bern experience in a subgroup of patients with severe acquired ADAMTS-13 deficiency. Semin Hematol 2004; 41: 48– 59.
Fujimura Y, Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara Medical University during 1998–2008. Intern Med 2010; 49: 7–15.
Furlan M, Robles R, Solenthaler M, Lammle B. Acquired deficiency of von Willebrand factor-cleaving protease in a patient with throm- botic thrombocytopenic purpura. Blood 1998; 91: 2839–46.
Jin SY, Skipwith CG, Zheng XL. Amino acid residues Arg(659), Arg(660), and Tyr(661) in the spacer domain of ADAMTS13 are crit- ical for cleavage of von Willebrand factor. Blood 2010; 115: 2300–10.
Klaus C, Plaimauer B, Studt JD, Dorner F, Lammle B, Mannucci PM, Scheiflinger F. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood 2004; 103: 4514–9.
Motto DG, Chauhan AK, Zhu G, Homeister J, Lamb CB, Desch KC, Zhang W, Tsai HM, Wagner DD, Ginsburg D. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest 2005; 115: 2752–61.
Peyvandi F, Lavoretano S, Palla R, Feys HB, Vanhoorelbeke K, Battaglioli T, Valsecchi C, Canciani MT, Fabris F, Zver S, Reti M, Mikovic D, Karimi M, Giuffrida G, Laurenti L, Mannucci PM. ADAMTS13 and anti-ADAMTS13 antibodies as markers for recur- rence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica 2008; 93: 232–9.
Rajewsky K. Clonal selection and learning in the antibody system. Nature 1996; 381: 751–8.
Zakarija A, Kwaan HC, Moake JL, Bandarenko N, Pandey DK, McKoy JM, Yarnold PR, Raisch DW, Winters JL, Raife TJ, Cursio JF, Luu TH, Richey EA, Fisher MJ, Ortel TL, Tallman MS, Zheng XL, Matsumoto M, Fujimura Y, Bennett CL. Ticlopidine- and clop- idogrel-associated thrombotic thrombocytopenic purpura (TTP): review of clinical, laboratory, epidemiological, and pharmacovigilance findings (1989–2008). Kidney Int Suppl 2009; 75: S20–4.
Zhang X, Halvorsen K, Zhang CZ, Wong WP, Springer TA. Mech- anoenzymatic cleavage of the ultralarge vascular protein von Wille- brand factor. Science 2009; 324: 1330–4.
Appendices
Appendix 1 – Details on reagent preparation
Recombinant human ADAMTS13 (rh-ADAMTS13)
Assay each batch by ADAMTS13 antigen assay and aliquot in amounts appropriate to the volume required for the assay runs. For use, thaw at 37C and dilute to 5g/ml in PBS.
Tween 20 sigma-Aldrich
Phosphate buffered saline (PBS) Sigma-Aldrich tablets
Dissolve 5 tablets in 1L deionised water
Bovine Serum Albumin (BSA) Sigma-Aldrich
PBS: BSA
Dissolve 2g BSA in 100mL PBS (2% BSA)
PBS Tween Wash Buffer
Add 1mL Tween 20 to 1L PBS (0.1% Tween)
Rabbit anti-human IgG polyclonal HRP conjugate
Dilute 1:1000 in PBS: BSA (12L in 12mL) immediately before use
0.1M Citrate Phosphate buffer pH 5.0
23.87g di-Sodium Hydrogen orthophosphate dodecahydrate
7.30g Citric Acid anhydrous
Make up to 900mL with deionised water. Adjust pH with 4M Sodium Hydroxide (pH 5.0), bring to 1L with deionised water and must be stored at 4C.
1, 2-ortho phenylene diamine dihydrochloride (OPD) 15mg tablets
Hydrogen Peroxide 6%
Sulphuric Acid 2M (Concentrated H2SO4)
Appendix 2 – Details on method including calibration
- Make up 12mL rh ADAMTS13 at 5g/mL in PBS
- Coat plates with 100l per well, apply seal and incubate at 4C overnight
- Wash plates 3 times with 250 PBS: Tween (Coated/Washed plated are stored at -80 freezers for future use. The plate was reached to room temperature before it was used)
- Block plate with 200 PBS: BSA for 1 hour at room temperature
- Thaw test plasma samples (38 per plate), index plasma and quality control for plasma at 37C for 20 minutes
- Prepared a standard curve by diluting the Index Reference Plasma in PBS: BSA to achieve 100, 80, 40, 20, 10, 5 and 0% concentration. The 80% standard curve should be prepared by diluting 160L plasma with 40L PBS: BSA. The other dilutions may be prepared by diluting 100L of the 80% standard with 100L PBS: BSA in a series of polypropylene test tubes. PBS: BSA buffer is used for the 0% standard
- Dilute all standards, test samples ad QC plasmas. 1:100 in PBS: BSA (10L samples with 990L buffer)
- Wash plate 3 times with 250L PBS: Tween
- Add 100L diluted standards, test or QC samples to duplicate wells on the microplate according to the plate plan and incubate at room temperature for 1 hour
- Wash plate 3 times with 250L PBS: Tween
- Prepare the diluted rabbit anti-human IgG polyclonal HRP conjugate (12L antibody in 12ml PBS: BSA, 1/1000) and add 100L to each well. Incubate at room temperature for 1 hour
- Prepare peroxidase substrate, just before use: 15mg OPD + 24ml Citrate phosphate buffer + 12L 6% hydrogen peroxide
- Wash plate 3 times with 250L PBS: Tween
- Add 100L fresh substrate to each well and observe plate on the bench until optimum colour development has occurred ~5 minutes (as indicated by a graduation of colour through the standard curve)
- Add 50L 2M sulphuric acid to each well to stop the reaction
- Read plate at 490nm within 10-12 minutes
Appendix 3 – Results
Table 5. Results from 50 normal patients using the di-sodium Hydrogen orthophosphate dodecahydrate buffer
| Sample Number | Normal | Sample Number | Normal |
| 1 | 4 | 26 | 4 |
| 2 | 5 | 27 | 5 |
| 3 | 2 | 28 | 3 |
| 4 | 2 | 29 | 4 |
| 5 | 2 | 30 | 2 |
| 6 | 1 | 31 | 2 |
| 7 | 3 | 32 | 4 |
| 8 | 4 | 33 | 2 |
| 9 | 1 | 34 | 4 |
| 10 | 1 | 35 | 3 |
| 11 | 2 | 36 | 1 |
| 12 | 2 | 37 | 1 |
| 13 | 5 | 38 | 1 |
| 14 | 4 | 39 | 1 |
| 15 | 3 | 40 | 2 |
| 16 | 4 | 41 | 2 |
| 17 | 3 | 42 | 2 |
| 18 | 3 | 43 | 2 |
| 19 | 2 | 44 | 1 |
| 20 | 1 | 45 | 4 |
| 21 | 1 | 46 | 5 |
| 22 | 3 | 47 | 3 |
| 23 | 2 | 48 | 2 |
| 24 | 1 | 49 | 1 |
| 25 | 3 | 50 | 1 |
Table 6. Results of 30 patients with positive Lupus screening using the di-sodium Hydrogen orthophosphate dodecahydrate buffer
| Sample
Number |
Lupus
|
Sample Number | Lupus | ||
| Dodecahydrate | Anhydrous | Dodecahydrate | Anhydrous | ||
| 1 | 12 | 5 | 16 | 8 | 5 |
| 2 | 2 | 2 | 17 | 2 | 1 |
| 3 | 2 | 2 | 18 | 4 | 4 |
| 4 | 2 | 2 | 19 | 2 | 2 |
| 5 | 8 | 5 | 20 | 7 | 3 |
| 6 | 5 | 4 | 21 | 1 | 1 |
| 7 | 5 | 3 | 22 | 1 | 1 |
| 8 | 13 | 4 | 23 | 9 | 5 |
| 9 | 10 | 5 | 24 | 3 | 3 |
| 10 | 6 | 6 | 25 | 8 | 5 |
| 11 | 9 | 3 | 26 | 2 | 2 |
| 12 | 4 | 1 | 27 | 3 | 1 |
| 13 | 2 | 1 | 28 | 0 | 1 |
| 14 | 7 | 7 | 29 | 8 | 1 |
| 15 | 1 | 1 | 30 | 1 | 1 |
Table 7. Results of 20 patients with positive syphilis screening using the di-sodium Hydrogen orthophosphate dodecahydrate buffer
| Sample
number |
Syphilis
|
Sample
Number |
Syphilis | ||||
| Dodecahydrate | Anhydrous | Dodecahydrate | Anhydrous | ||||
| 1 | 13 | 5 | 11 | 5 | 5 | ||
| 2 | 4 | 4 | 12 | 2 | 2 | ||
| 3 | 2 | 3 | 13 | 2 | 2 | ||
| 4 | 8 | 4 | 14 | 2 | 3 | ||
| 5 | 2 | 1 | 15 | 1 | 0 | ||
| 6 | 8 | 8 | 16 | 7 | 5 | ||
| 7 | 9 | 5 | 17 | 3 | 2 | ||
| 8 | 2 | 1 | 18 | 8 | 3 | ||
| 9 | 1 | 0 | 19 | 1 | 0 | ||
| 10 | 6 | 6 | 20 | 1 | 0 | ||
Table 8. Results of the 12 patients showing pre-and post-plasma transfusion
| Sample number | PRE | POST |
| 1 | 41 | 10 |
| 2 | 10 | 3 |
| 3 | 24 | 4 |
| 4 | 20 | 4 |
| 5 | 40 | 10 |
| 6 | 51 | 4 |
| 7 | 30 | 5 |
| 8 | 63 | 24 |
| 9 | 11 | 2 |
| 10 | 10 | 5 |
| 11 | 41 | 6 |
| 12 | 7 | 5 |
Figure 10. Microwell plate used for the assay

Figure 11. F96 Maxisorp Nunc-Immuno Plates used to read the plate at 490nm
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medical"
The word Medical refers to preventing or treating injuries or illnesses, relating to the study or practice of medicine. Medical care involves caring for a patient and helping them through their journey to recovery.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




