Thalidomide Disaster: Have Medical and Pharmaceutical Industries Learnt from Past Mistakes?
Info: 10936 words (44 pages) Dissertation
Published: 30th Dec 2021
Tagged: HistoryMedicinePharmacology
Following the thalidomide disaster: Have the Medical and Pharmaceutical industries learnt from factors that contributed to past mistakes?
Abstract
A report exploring and evaluating how the pharmaceutical and medical sector are dealing with factors that contributed to the Thalidomide Tragedy which blighted 100,000+ lives.
Contents
Background
Off-label Prescription and Thalidomide
Animal Testing and Thalidomide
Vioxx Scandal and thalidomide
South America and Thalidomide
Legislation and thalidomide
Regulations in the USA – The Federal Drugs Administration (FDA)
Regulations in the UK – The Medicines and Healthcare Products Regulatory Agency (MHRA)
Regulations in West Germany
Conclusion
Reference List
Bibliography
Background
From 1959-1964 a drug known by its common chemical name – thalidomide, wreaked havoc in at least 46 countries. It is estimated that 20,000 children and infants were born with birth defects such as phocomelia. Phocomelia refers to a birth defect in which “the upper portion of a limb is absent or poorly developed, so that the hand or foot attaches to the body by a short, flipperlike stump.” (The American Heritage® Medical Dictionary, n.d.).

Thalidomide caused several miscarriages such that if they were factored in, the number of ‘lives’ affected rises to about 100,000. There are only about 5,000 survivors in the world today (Lingham, 2000) (Miller, 1991).
In the West, thalidomide did not directly affect the USA because it was never approved by the Federal Drugs Administration (FDA) officer in charge – Francis Kelsey. Per Kelsey, she stated that “deficiencies in all areas were found during the initial review and in several subsequent resubmissions”. Kelsey was predominantly concerned about the report of peripheral neuritis as a side effect, of which the FDA were only informed in February 1961 despite its acknowledgement in Britain in September 1960. The valid concerns raised by Kelsey delayed the approval of thalidomide until its adverse effects were found and it was removed from the Australian and European markets in November 1961 (Botting, 2015).
The drug, developed by Chemie Grünethal, was initially hailed as a sedative but was found to be effective when given to pregnant mothers to ease morning sickness. But as of November 1961, it was pulled off the markets of the UK and West Germany. Thalidomide soon became known as a notorious killer and disabler of thousands of babies (Miller & Stromland, 1991).
When thalidomide was taken during pregnancy (particularly during a specific window of time in the first trimester), it caused startling birth malformations, and death to children. Any part of the foetus that was in development at the time of ingestion could be affected (Knapp, et al., 1962).
For those babies who survived, birth defects included: deafness, blindness, disfigurement, cleft palate, many other internal disabilities, and of course the disabilities most associated with thalidomide: phocomelia (Botting, 2015).
The teratogenic effects of the drug and peripheral neuritis it causes were not picked up for a number of reasons. These factors that contributed to the disaster are subject of evaluation and exploration by many academics.
The following report explores factors that played an important role in the thalidomide disaster and how the pharmaceutical industry in partnership with the medical sector has learnt from these past mistakes.
Off-label Prescription and Thalidomide
While there is some ambiguity into the nature of the original clinical trials given that the files from the company that developed it, Chemie Grünenthal were destroyed shortly after the scandal (Stephens & Rock, 2001), it is often suspected that the drug was prescribed off-label (Thompson, 2016) by doctors. Off-label prescription is the practice of prescribing drugs for a purpose outside the scope of the drug’s approved label (Miller, 2015).
Some speculate that thalidomide was “widely prescribed off-label in the U.K. in the 1960s for alleviating morning sickness” (Thompson, 2016) before its teratogenic effects were discovered. Teratogens are any agent which can cause congenital abnormalities to a foetus (The American Heritage® Medical Dictionary, 2007). It is likely that thalidomide is a case of off-label prescription. The way in which the drug made its way onto the market suggests this. Thalidomide was “first recommended for the treatment of epilepsy (a disease of the nervous system causing a person to fall unconscious)” (Department of Curriculum and Instruction, Chinese Unversity of Hong Kong, 2007). It was then seen that the drug had little to no effect on epilepsy and related conditions and was instead re-developed and prescribed as a sedative which Chemie Grünenthal claimed to relieve “anxiety, insomnia, gastritis, and tension” (Miller, 1991). Shortly after, the drug was seen to alleviate morning sickness and was “deemed to be so safe” (British Broadcasting Corporation, 2005) as a sedative that it was heralded as the drug of choice to treat morning sickness. Therefore, the drug was being used for uses other than what it was approved for. The problem with this and most off-label prescriptions is that the target audience accounted for in the original clinical trials would not have been pregnant women. This suggests that doctors and physicians were unaware of the drug’s effects on this percentage of the population, given that there was no clinical data to support the anecdotal findings that thalidomide helped in relieving morning sickness. The practice of off-label prescription leaves room for “more prevalent occurrence of unanticipated, and often serious, adverse drug reactions” (Bara, et al., 2009). These adverse reactions such as the teratogenic effects of thalidomide are one of the reasons why off-label prescriptions are potentially dangerous and why it is attributed as one of the factors that contributed to the thalidomide scandal.
However, off-label prescription is still a common practice today post-thalidomide. This is because off-label prescription of drugs can be positive as it gives patients who have exhausted all other approved options to treat life-threatening diseases. It is especially beneficial for cancer patients as a drug “approved for one type of cancer may actually target many different tumours” (Miller, 2015).
Another example of off-label prescription is with the treatment of chronic pain using antidepressants. In most ways, this cannot be seen as another mistake akin to thalidomide given that unlike thalidomide, the drugs were tested before being used in its off-label fashion. Thalidomide was very likely prescribed and given to pregnant mothers based on anecdotal evidence, concrete scientific research was not done into it to explore the positives of the drug in the new target audience that it was going to be prescribed for – pregnant women. While there is some debate as to whether the drug was tested for teratogenic effects in the original clinical trials, it is plausible that the audience of ‘pregnant women’ were never fully explored, as they were not the primary audience the drug was targeted to. This is considering that it was originally a drug synthesised as a sedative for the general populace. Again, there is some ambiguity as records of original clinical trials are unavailable (Stephens & Rock, 2001).
The off-label prescription of antidepressants used to treat chronic pain is subject to widespread research. Where thalidomide might have been prescribed off-label without proper testing after discovery of its anti-morning sickness effects (Department of Curriculum and Instruction, Chinese Unversity of Hong Kong, 2007) the use of antidepressants to treat chronic pain is subject to extensive randomised “controlled clinical trials, mostly in patients with post-herpetic neuralgia, painful diabetic neuropathy, and central pain, (Ryder & Stannard, 2005). There is significant evidence showing the benefit of antidepressants. As noted in the journal ‘Continuing Education in Anaesthesia, Critical Care & Pain’ (Ryder & Stannard, 2005) these drugs “to varying degrees, block several other receptor types involved in pain … it may also have blocking effects on calcium and sodium channels and be weakly stimulatory at μ-opioid receptors”. These scientific reports translate to the beneficial factors that the antidepressants have on the body tissues and proteins.
This indicates that extensive research went into the off-label prescription of antidepressants for chronic pain before it was prescribed to patients. The trials were done on the appropriate target audience, namely those with “post-herpetic neuralgia, painful diabetic neuropathy and central pain” (Ryder & Stannard, 2005) . Therefore, any results, which show a “benefit of antidepressants”, are reliable as they are to the right target audience. This could suggest that drug companies are now synthesising drugs based on science rather than anecdotal findings as happened in thalidomide.
Although off-label prescription to treat chronic pain has been fully explored, this is not always the case. An example where off-label prescription has led to a poor outcome post-thalidomide is the Fen-Phen case. The Fen-Phen case involves two drugs (fenfluramine hydrochloride and phentermine hydrochloride) which the FDA approved individually as short-term treatments for obesity (Miller, 2015). The two drugs gained popularity shortly after an article in a medical journal describing the drug cocktails as a tool for dramatic weight loss. Soon after, doctors prescribed both drugs together. The result was devastating. Many patients ended up with severe heart valve damage, some potentially fatal. After a multi-billion-dollar lawsuit, the FDA ordered Fen-Phen off the market.
Arguably with this case and others like it, off-label prescription should be tighter controlled since it is very likely an important factor into how thalidomide got on the market. In actuality, off-labelling is loosely controlled and only a “few countries such as the USA and France have taken an initiative and have come up with the regulations about off-label use of medicine” (Gupta & Nayak, 2014).
Regulations in the UK and the USA allow physicians to prescribe off-labelled medication to patients without letting patients know the drug is prescribed outside of its approved use. American legislation states “doctors are free to prescribe a drug for any reason [they think is medically appropriate] and are not required to tell a patient that the drug is being used off-label” (Miller, 2015). The UK regulations state similarly that “healthcare professionals can prescribe unlicensed medicine” with an added caveat that “the responsibility that falls on healthcare professionals when prescribing a medicine off-label may be greater than when prescribing a licensed medicine within the terms of its license” (Medicines and Healthcare Products Regulatory Agency, 2009). Perhaps if expectant mothers were told that the drug they were prescribed was being done so outside of its approved use, the thalidomide case may have turned out differently.
In this aspect, the pharmaceutical sector has continued with the previous practices that were in use as at thalidomide. Apart for the USA and France which have obscure legislation about how off-label drugs can be used, other countries such as the UK have had no changes in legislation regarding off-label prescription (Medicines and Healthcare Products Regulatory Agency, 2009) . This is despite the role that off-label prescription played in thalidomide. The pharmaceutical sector does not appear to have considered the role off-labelling played in Thalidomide. There is little evidence to suggest that attempts are currently being made to test off-label drugs thoroughly before giving them to fragile percentages of the population such as pregnant women (Gupta & Nayak, 2014).
Off-label prescription is a double-edged sword, while it may be very useful for some patients it can also “expose them to unrestricted experimentation, unknown health risks, or ineffective medicine” (Botting, 2015). Hence, to avoid another disaster caused by the misuse of off-label drugs, there is an urgent need for guidance to encourage proper off-label use of medicine by the distribution of scientifically valid and authentic information from the pharmaceutical companies (Gupta & Nayak, 2014).
Animal Testing and Thalidomide
It is hard to talk about the thalidomide case without mentioning animal testing. The reasons range from the arguably negative effects that animal testing played to the subsequent changes in legislation that followed thalidomide including the West German Drug Law which is described as the “the single most important consequence of the thalidomide disaster” (Grunenthal, 2014).
As is usual of any topical issue, the reasons that contributed to the thalidomide disaster have been evaluated to a great degree. Some argue that thalidomide highlights the failures of animal testing. Arguably, since “extensive animal testing was done” (Grunenthal, 2014) and as was confirmed by Timemagazine February 23, 1962, thalidomide was released “after three years of animal tests”, evidence suggests that animal testing could not highlight the teratogenic effects of thalidomide. Teratogenicity refers to the toxicity of a substance on embryos. Even the company who developed and marketed thalidomide, Chemie Grünenthal (now Grünenthal) confirm that they tested the active drug substance thalidomide on “certain animals in accordance with standard pharmaceutical industry practice at the time” (Grunenthal, 2014).
In fact, the animal testing was so extensive that as per James L. Schardein, an expert in teratogens (birth defect-causing substances), “In approximately 10 strains of rats, 15 strains of mice, 11 breeds of rabbits, 2 breeds of dogs, 3 strains of hamsters, 8 species of primates and in other such varied species as cats, armadillos, guinea pigs, swine and ferrets in which thalidomide has been tested, teratogenic effects have been induced only occasionally” (Schardein, 1976).
Several academic journals testify that enough animal testing was done such that based on the results, thalidomide was thought to be safe. As per H. Taussig, a chemist who researched thalidomide, “Grünenthal has tried to reproduce phocomelia in rats, mice, and rabbits and has failed, in Keil the drug was fed to hens and the chicks were normal” (Taussig, 1962). This indicates that despite the vast numbers of animal testing trials that were carried out (Schardein, 1976), the problem in question was not the merely the ‘number’ of animal tests carried out but that animal testing could not in its nature recognise the teratogenic effects of thalidomide.
Some pro-animal testers argue that the problem was not due to the ‘number’ of testing but rather that because of the fewer advancements in technology, teratogenicity tests were not carried out. The UK-based group ‘Understanding Animal Research’ state that foetal effects from maternal ingestion, was unfamiliar pre-thalidomide and that had thalidomide been tested on pregnant animals: “… the same birth defects would have shown up in the animals – as they did subsequently – and thalidomide would never have been used by pregnant women.” (Greek, et al., 2011). Their argument lies that if teratogenicity tests had been carried out, “the teratogenic effects would have been caught” (Fulkerson, 2011). The problem with this argument is twofold. For one, there is an extremely high likelihood that teratogenicity tests were carried out and even if they had it is unlikely animal testing would have picked up the teratogenicity of the drug.
Testing for teratogenicity was a common practice in the pre-thalidomide era. Review articles in the 1950s and early ‘60s when the scandal was newly publicised traced the origins of teratology testing. Evidence shows that teratogenic tests were common place since about 1891 when Darestet experimented with chick embryos and induced congenital defects” (Greek, et al., 2011).Even critically acclaimed chemist and Nobel laureate Roald Hoffman observed that “Indeed, animal testing for teratogenicity of new drugs was routine in the major pharmaceutical companies. Hoffmann-LaRoche’s Roche Laboratories published a major reproductive-system study of its Librium in 1959. Wallace Laboratories did so for Miltown in 1954. Both incidents antedate the thalidomide story” (Hoffman, 1995).
Therefore, while we may never know the truth of the tests that were done given that Grünenthal records were destroyed (Stephens & Rock, 2001), it is highly likely that teratogenicity tests were done. The argument that suggests the disaster would have been averted if pregnancy animals were used as claimed by groups such as Pro-Test is null.
It cannot be overlooked however that even if teratogenic tests had been done, which they likely were, the tests would not have picked up the teratogenic effects of thalidomide. With thalidomide, it is only possible to produce specific deformities in very few animal species. In this case, therefore, it is “unlikely that specific tests in pregnant animals would have given the necessary warning: the right species would probably never have been used” (Teeling-Smith, 1980).
Animal testing would not have picked up the teratogenicity effect on humans because the dose detected to cause phocomelia in the New Zealand white rabbit (which was the first species in which phocomelia was observed) were “only at a dose between 25 to 300 times” (Safer Medicines, 2015) that given to humans. This means thalidomide would still have been approved for sale because the toxic dose was too large for normal human consumption and because a far larger majority of other species tested were not affected (Safer Medicines, 2015). This is still how most drugs tested with animals are evaluated today.
Ideally, knowing what we know now, it is useful to question how pharmaceutical companies test thalidomide or any other drug to assure safety in pregnant women. The short answer is that such testing is currently impossible. Per (Lo & Friedman, 2002) more than 90% of all the drugs approved since 1980 have not been adequately tested for teratogenicity despite the thalidomide case. The risk from such drugs is listed as “undetermined”. Given the scatter-gram effect of thalidomide in terms of producing congenital abnormalities in different species and the fact that no combination of animals has since been shown to be predictive for teratogenicity in humans, it can be concluded that even if thalidomide were extensively tested today on animals using the best science currently available, it would still be labeled Category C (category C refers to drugs in which the effects are “undetermined”) (Greek, et al., 2011). This highlights that animal testing cannot predict all the adverse effects of a drug and other methods ought to be used.
Pro-Animal testing groups claim the opposite. They argue that animal testing must be effective as despite the constant use of animal testing in drug development today, another disaster akin to thalidomide is yet to occur. This is incorrect. Although there has not been another large-scale disaster regarding birth defects, other drug disasters have occurred in which animal testing played an important role. The reason society has not seen another thalidomide disaster lies not in the fact that all drugs are tested on animals but rather that new drugs are rarely given to pregnant women (Greek, et al., 2011). Currently, the only way to determine for certain whether a drug is a teratogen is through epidemiology. Better monitoring of medication intake could facilitate establishing a safety profile (Greek, et al., 2011).
Since pregnant women are no longer prescribed drugs due to Sellers’ (a respected physician) advice not to administer drugs to pregnant women without extremely good reason or if the condition is not life-threatening (Seller, 1962) . Perhaps in this way the medical and pharmaceutical industries have learnt from the thalidomide disaster when it comes to prescribing drugs to such a fragile target audience after all. It would still appear that clinical trials are still not good enough and are far dependent on animal testing too strongly which has allowed other disasters, though not to do with teratogenicity, to occur again such as the Vioxx fiasco and the Paraxel case.
Vioxx Scandal and thalidomide
Thalidomide was a tragedy labelled as one of the “darkest episodes in pharmaceutical history” (Drug Watch, 2014) but the Vioxx fiasco has been described as the “the worst disaster in drug history” (Drug Watch, 2014). Interestingly, Vioxx’s recall happened post-thalidomide some 43 years after thalidomide was also recalled. After a lot of broken promises regarding justice for thalidomide, a disaster that many victims had expected to be a once-in a generation occurrence, less than 50 years later another scandal much worse and more fatal claimed 38,000 lives and was received by 25 million right under the FDA’s watch.
On September 30, 2004, the drug company Merck recalled its pain killer under the brand name Vioxx (also known as rofecoxib) from the market because it was found to increase the risk of blood clots in patients. Evidence suggests that human observational data predicted these effects as early as 1996, and human clinical data confirmed the danger in 2001 (Drug Watch, 2014) (Evans, et al., 2011). The manufacturers allegedly chose instead to use animal-testing data which showed a more positive view of the drug and allowed it to reach the market. Thus, Vioxx and thalidomide are similar in that they highlight the inaccuracy of animal testing regardless of how rigorous and extensive the testing is.
Post-thalidomide, the regulation of drug testing was tightened considerably and systems introduced to report adverse reactions. However, despite this the Vioxx case illustrates that it was fallible. Vioxx and related drugs were supposedly good painkillers with fewer side-effects than existing drugs. It was launched in 1999 but almost immediately some researchers questioned its safety. It has been estimated that Vioxx caused 88,000–139,000 heart attacks, with about 40% of them terminal. Some critics accused the FDA of not doing enough to protect patients. Merck was accused of manipulating data (which it denies). Merck denies liability and a settlement of nearly $5billion has since been agreed for Vioxx lawsuits (Evans, et al., 2011).
Vioxx highlights the inaccuracy of animal testing as confirmed by a report published by the Physicians Committee for Responsible Medicine (PCRM). The report reveals that “Vioxx and other COX-2 drugs had a heart-protective effect in mice and other animals” which is precisely the opposite of how the drugs later reacted in humans (Pippin, 2005). This and other similar data were presented to the FDA which spurred its approval as the drug appeared beneficial. The report also reveals that once clinical trials started showing that the drugs caused heart problems in humans, the pharmaceutical company ignored this information and instead pointed to the animal tests as “evidence” that the drugs were safe. Writer of the PCRM report, Dr Pippin, illustrates that the Vioxx animal testing debacle is not unique. Over the years, millions of patients have been exposed to harmful drugs, such as Rezulin and Baycol, that seemed safe in tests on mice, dogs, rats, monkeys, horses, and other animals. “Reliance on animal tests enabled the FDA to approve Vioxx,” says Dr Pippin, “It is time to turn to newer, more reliable human-based methods such as studying drug metabolism using human liver subcellular fractions” (Pippin, 2005).
Vioxx and thalidomide are similar in this way given that both disasters can be attributed to the inaccuracy (not extensiveness) of animal testing. Unlike thalidomide, Vioxx trials proceeded on to human clinical trials. Arguably this is due to a difference in era as human clinical trials were uncommon when thalidomide was being manufactured. The human clinical testing did not prevent the Vioxx scandal despite evidence showing an increase in heart attacks. The data was allegedly side-stepped and positive animal-testing data was used. It concludes that post-thalidomide, the pharmaceutical industry may have stepped-up testing to avoid another drug disaster but some companies in the sector may be unwilling to use negative results that bar the drug from the market and prevent sales. Big Pharma may be learning from the thalidomide mistake, but has it become more corrupt in the process? (Stephens & Rock, 2001).
However, this may not be a new fad; thalidomide had a similar story while on the market. When evaluating the history of Vioxx, the historical tale of the duplicity and injustices associated with the effects of thalidomide become starkly prescient (Stephens & Rock, 2001). Like Vioxx, thalidomide executives were allegedly aware of the effects of thalidomide prior to public outcry for its recall.
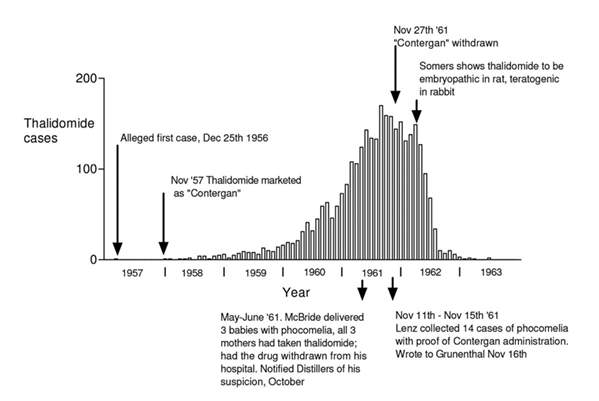
Figure 1 ”Columns represent the monthly incidence in Germany of births of children deformed because of ingestion of thalidomide by the mother. Data was collected by the meticulous retrospective epidemiological studies of Widukind Lenz” (Botting, 2015)
Before thalidomide was marketed in November 1957 under the brand name Contergan in West Germany (Botting, 2015), in October of that year, a neurologist Dr Voss identified polyneuritis in 3 patients who had taken Contergan( the brand name for thalidomide) for a year, and “raised with Grünenthal his concern that the drug might have a toxic action on peripheral nerves”. Supposedly, from April 1959, Grünenthal representatives had also received information from chemists and doctors that Contergan caused several side effects including abnormally cold hands and feet, paraesthesia, and giddiness. In response, Grünenthal replied to Voss saying, “no such side effects have come to our notice” thereby keeping the drug on the market (Botting, 2015).
Nonetheless, Voss submitted his findings at a neurological congress in Dusseldorf in April 1960. This prompted another neurologist Frenkel to submit a paper to a journal ‘Medizinische Welt’ describing 20 patients he believed to have ‘thalidomide induced peripheral neuritis’. Grünenthal representatives attempted to persuade Frenkel to withdraw or delay publication but he refused. Shortly after, in a letter to the British Medical Journal of December 31st, 1960, entitled “Is thalidomide to blame,” Dr A. Leslie Florence from the UK described 4 patients with signs of marked paraesthesia (a skin sensation, such as burning, prickling, itching, or tingling, with no apparent physical cause) which affected first the feet and then the hands (The American Heritage® Medical Dictionary, 2007). Patients also observed coldness of the extremities and nocturnal cramp. All patients had been taking thalidomide for between eighteen months to two years. Termination of the drug resulted in alleviation but not removal of the symptoms (Botting, 2015).
By the end of May 1961 there were at least 1,300 cases of peripheral neuritis associated with long-term thalidomide therapy and Chemie Grünenthal were forced to take steps to have the drug supplied on a prescription-only basis. This action in comparison to the catastrophic effects soon to follow due to its undiscovered teratogenicity seem almost insignificant. (Botting, 2015) (Stephens & Rock, 2001).
From the evidence, it can be concluded that both drugs were subject to cover-ups by respective executive officials. While with thalidomide, Chemie Grünenthal knew of the effects shortly after the drug had been released, the Vioxx officials knew of the effects before they applied to the FDA for their distribution license.
Despite the mistakes made in thalidomide, Merck as part of the pharmaceutical industry did not learn or perhaps want to learn from thalidomide. Both drugs were kept on the market by both companies for sales and revenue.
Both disasters also share the same lineage in that they occurred due to inaccurate data from animal testing. The complaint filed in Atlantic County, New Jersey, asserts that reliance on the animal data by Merck was “grossly improper and a violation of Merck’s legal and ethical obligations.” Despite having test results “from over 8,000 humans that the drug was killing people,” Merck continued to use animal studies to justify continuing to sell Vioxx (Pippin, 2005). The Vioxx fiasco is arguably more sinister than thalidomide in that accurate human data was available but was allegedly ignored putting 25 million lives in danger.
The Vioxx scandal shows that, more than 50 years after thalidomide, there is still a lot to do to make sure that that medicines are tested properly, that the process is transparent to all parties and that there is strong evidence to prove efficacy and safety. As a group of academics put it, ‘our system depends on putting patients’ interests first. Collaborations between academics, practising doctors, industry, and journals are essential in advancing knowledge and improving the care of patients. Trust is a necessary element of this partnership, but the recent events have made it necessary to institute proper systems that protect the interests of patients. A renewed commitment by all those involved and the institution of these systems are the only way to extract something positive from this unfortunate affair’ (Evans, et al., 2011).
South America and Thalidomide
Following the discovery by Israeli physician Jacob Sheskin on the positive effects of thalidomide on a patient who exhibited erythema nodosum leprosum (ENL) – a painful skin condition which is a complication of leprosy – in 1964, (Silverman, 2002) Brazilian physicians have been using thalidomide as the drug of choice to treat severe ENL since 1965.
Following this, by 1996 at least 34 cases of thalidomide embryopathy were recorded in people born in Brazil after 1965 (Castilla, et al., 1996). Embryopathy refers to the abnormality of an embryo or foetus usually caused by an agent e.g. thalidomide (Merriam-Webster.com, n.d.). The cases of thalidomide embryopathy underscore a problem of controlling access given that new cases of thalidomide embryopathy have occurred when the drug is used for medical conditions, especially regarding leprosy (Castilla, et al., 1996). Supposedly, the production, dispensing, and prescription of thalidomide is strictly controlled since 1994, through the S.T.E.P.S. programme. The programme requires women to use two different types of birth contraception and report back for regular pregnancy tests (Paumgartten & Chahoud, 2006). Despite this, cases of thalidomide induced embryopathy continue, with at least 100 cases identified in Brazil between 2005 and 2010 (Crawford, 2013).
In July 1998, the FDA in the USA approved thalidomide for treatment of ENL (Department of Curriculum and Instruction, Chinese Unversity of Hong Kong, 2007). Thalidomide does not kill the bacteria that cause leprosy instead it changes the body’s natural immunological response to the bacteria by decreasing the levels of alpha tumour necrosis factor (A-TNF), which is the factor primarily responsible for tissue inflammation in ENL patients (Department of Curriculum and Instruction, Chinese Unversity of Hong Kong, 2007).
While thalidomide is beneficial in this way, yet again regulations are slack. This time instead of the weak regulations concerning a lack of proper teratogenic testing, off-label prescription, or evaluation of the proper effects of the drug on the fragile target audience it was aimed at, it is to do with the distribution of the drug after the original disaster. In South America, “regulatory authorities granted exemption from licensing requirements to enable doctors to obtain limited supplies of thalidomide” (Pannikar, 2003). Authorities are said to have put the distribution “under strictly controlled circumstances for use in named patients” but the “controlled circumstances” appear inefficient as unfortunately some of the pills have become available to pregnant women. This has led to the rise of infants born with phocomelia and other adverse effects that indicate thalidomide embryopathy (Castilla, et al., 1996). There were 34 cases of disfigurement in which thalidomide is confirmed as the primary cause.
There is some question as to why thalidomide has been the drug of choice to treat ENL since the “WHO (World Health Organisation) does not recommend” it (Department of Curriculum and Instruction, Chinese Unversity of Hong Kong, 2007) (Castilla, et al., 1996).There are other options for treatment of ENL and there are strong arguments on both sides to retract thalidomide for treatment of ENL. It is however not the fact that thalidomide is used to treat ENL but rather that the reintroduction, despite its supposed use in “controlled circumstances”, opens a “problem of controlling access” to undesired target audiences (Castilla, et al., 1996).
There is merit in this argument given that there are other modalities to treat ENL, which are not teratogenic. It would seem that the reintroduction of thalidomide is a simply a gateway to reintroduce thalidomide for other conditions (Pannikar, 2003). Another drug, prednisolone is more effective in controlling ENL and neuritis. In addition, clofazimine, an anti-leprosy drug, produced in the ‘60s has the anti-inflammation action needed to treat ENL. Studies showed that clofazimine is the drug of choice for the management of chronic, recurrent ENL reactions as it had both anti-inflammatory reaction and anti-leprosy effect (Pannikar, 2003). Moreover, while almost all patients given thalidomide relapsed after discontinuation of the drug, none of the patients treated with clofazimine for ENL reactions relapsed (Castilla, et al., 1996). It is determined that clofazimine is so effective that it is now a component of the multidrug therapy (MDT). WHO introduced MDT in 1981 as the standard treatment for leprosy. The presence of clofazimine in the combination has significantly reduced the frequency and severity of ENL reactions worldwide (Pannikar, 2003).
Today ENL reaction is a rare complication, limited to a small proportion of multibacillary patients (patients with diseases relating to bacilli which are a type of bacteria). Most of the ENL reactions are mild in nature and do not require any specific treatment except with some analgesics/antipyretics. In those suffering ENL associated neuritis, the drug of choice is prednisolone. For chronic recurrent reactions, the drug of choice is clofazimine. It is clear that there are other very effective drugs (prednisolone and clofazimine) which are viable alternatives to treat ENL. It can be concluded that if the primary reason for reintroducing thalidomide is for the treatment of ENL, then thalidomide should be removed off the market. Experts at WHO claim, “there is no place for thalidomide in leprosy”. However, since thalidomide is cheap and easy to manufacture it takes precedence over the other modalities that can cure ENL (Department of Curriculum and Instruction, Chinese Unversity of Hong Kong, 2007). This is the main reason it is often used in the treatment of ENL in many countries. But the poignant question to ask is – does the cost of treatment outweigh the potential risks it can cause? However, regulations need to be tighter. There are strong advocates on both sides of the debate but there is no question that great care must be taken if thalidomide is given to a woman of childbearing age regardless.
The World Health Organization has recommended that thalidomide be used only in postmenopausal women (Pannikar, 2003). However, the fact that children still are being born with thalidomide embryopathy shows how difficult it is to guarantee safe usage (Castilla, et al., 1996). Guidelines for use are available (Miller, 1991) and “are outlined in detail by Powell and Gardner-Medwin (’97)” (Miller, 1991). The ongoing cases of thalidomide induced embryopathy is limited to the Third World, where leprosy is more prevalent and drug control measures are weaker. However, new applications for thalidomide are being tested in clinical trials, and ECLAMC (Latin American Collaborative Study of Congenital Malformations) believes that it has reason to suspect that “the stage may be set for a new catastrophe as great as that of the early sixties” (Castilla, et al., 1996).
The world’s pharmaceutical industries may have promised that there would not be another disaster like thalidomide for a while but the loose control on such a dangerous drug begs to differ. Despite the guidelines for use which some say can justify the reintroduction of the drug, pediatricians have found that in Brazil and Argentina thalidomide is available outside of official health authorities and that it is also sold open market in public pharmacies (FFDN, 2008). These two countries, which manufacture and export thalidomide, have the largest cases of thalidomide embryopathy (FFDN, 2008). This begs the question that if a teratogen like thalidomide is allowed so freely in the open market of a country where a very high percentage of citizens are illiterate, what is to stop another thalidomide disaster? The pharmaceutical sector may not be paying much attention to the 100+ cases that have happened between 2005 and 2010 (Crawford, 2013) but without tighter regulations regarding distribution another thalidomide disaster may come knocking. Since thalidomide is also being sold under different brand names such as “Ectiluran, Sedalis, Sedin, Slip, Verdil” (Warren, 1999), even literate individuals who are aware of thalidomide’s history may fall prey as they do not know about the drug they are taking since it is under a different brand name. There have been calls for the drug to be sold under the name ‘thalidomide’ to avoid confusion to others and allow a degree of openness to patients about the dangers of the drug they are about to take (Crawford, 2013).
Legislation and thalidomide
Before the thalidomide tragedy, there was not much in the way of regulation that was tight enough to allow manufacturers to prove efficacy of drugs before being put on the market. Post thalidomide, this was a section of the pharmaceutical industry that was scrutinised severely, leading to many important reforms most of which are still in play today. Around the world, as is common after a high-profile tragedy bordering on crime, laws were called upon to be changed.
The following is an exploration of three countries whom thalidomide had a direct or passive effect on: West Germany, the UK, and the USA.
Regulations in the USA – The Federal Drugs Administration (FDA)
The USA was only passively affected by thalidomide because it was never approved by FDA medical officer, Francis Kelsey, who was responsible for evaluating thalidomide before its use in the USA. Her decision spared America as one of the few countries in the West that was not directly affected by thalidomide. The widespread dissemination of the fact that thalidomide caused nerve damage (of which Grünenthal initially denied) was partially responsible for alerting Frances Kelsey, that this supposed medicine hailed to be “so safe” (British Broadcasting Corporation, 2005) was potentially dangerous. Hence, the subsequent delay in the approval of thalidomide until after its teratogenic effects were established in Europe prevented a disaster for the USA (Botting, 2015).
The Amendment imposed guidelines for the process of drug approval in the US and required that a drug be safe as well as effective before it could be approved and marketed.
Post the thalidomide tragedy, on 7 February 1963 the American Congress passed the 1962 Kefauver-Harris Amendment in addition to the 1938 Food, Drug, and Cosmetic Act. The Amendment required that pharmaceutical companies prove efficacy prior to marketing. While the thalidomide problem is lined to safety, an element that was already part of regulations, the laws were changed to add proof of efficacy (Hooper, 2008). This meant that drugs had to be safe as well as effective before it was marketed and approved. The new legislation required that drug companies which planned to investigate a drug clinically had to provide the FDA with an inclusive outline of the studies they had carried out. Any information regarding preclinical studies, the number and qualifications of the clinical investigators, and the nature of the study, were a required feature in the documentations presented to the FDA. The company also had to monitor the progress of all clinical studies and report its findings while keeping adequate receipts of drugs and names of persons whom had received the drug; physicians were not required to do this prior to the 1962 Amendment. (Tantibanchachai, 2014)
Thalidomide also influenced the FDA’s creation of pregnancy categories – a ranking of drugs based on their effects on reproduction and pregnancy. This pregnancy rating and the S.T.E.P.S. programme later served as a stepping stone for the FDA’s response to isotretinoin (a prescription drug used to treat severe acne), which like thalidomide caused severe birth defects and prompted its manufacturer and the FDA to create a risk management programme to prevent foetal exposure (Tantibanchachai, 2014).
The Amendment also mandated that the FDA explicitly approve a new drug application before the developing company could announce the product for sale. Prior to the 1962 Amendment, if the FDA did not disapprove a drug application within six months, the drug would be automatically approved within the subsequent sixty days. This meant that cases where it took more than 60 days for the FDA to process, a potentially dangerous drug was marketed before explicit approval (Tantibanchachai, 2014). The Amendment reduced approval of FDA drugs annually from 46.2 pre-Amendment to 15.7. The Kefauver-Harris Amendment has given the FDA greater control than it did previously to regulate drug manufacturers and prevent more drug disasters.
However, some maintain that the 1962 drug Amendment is still interpreted wrongly as mandating animal testing, despite other toxicology technologies that can supersede animal testing technology. It has been shown conclusively that testing on human tissue in vitro could have predicted the danger that thalidomide posed (Safer Medicines, 2015) ( Animal Friends Croatia, 2001). In vitro testing refers to tests carried out in a test tube or culture dish outside of humans or other living organisms. In vitro testing, computer modelling, and other technologies are increasingly surpassing animal models in both accuracy and efficiency, but the USA has yet to adapt its regulatory process to acknowledge these changes. Vast expenditure has been spent on animal toxicity testing but disasters including Vioxx and other cases such as Parexel occur indicating that some drugs remain unsafe which is incitement for further disasters.
Overall, legislation wise, the USA has made great steps to learn from thalidomide with the 1962 Amendment a kick-starter that allows the FDA to have greater control than it did previously to regulate drug manufacturers and prevent more drug disasters.
Regulations in the UK – The Medicines and Healthcare Products Regulatory Agency (MHRA)
In the UK, some form of control of drugs has been in operation since 1316 when a code of quality was established by the ordinaces of the Guild of Pepperers of Soper Lane. Until the introduction of the Medicines Act in 1968 – post thalidomide – there was, however, no legislation in place controlling the introduction of new drugs in the UK which related to their safety in use. Until that time most of the legislation was related to quality of therapeutic materials and the control of substances known to be either poisonous or narcotic (Kirkwood & Longley, 1994). In the 1950s while thalidomide was being developed and when it was on the market, there was very little control over the way therapeutic medicines were manufactured and marketed across Europe. The legislations regarding medicines were insubstantial with a fragmented series of laws which dealt briefly with the quality and promotion of drugs which combat diseases without evaluation for the proof of efficacy and safety (BBC Panorama, 2004).
The thalidomide problem shook the confidence of the public in the pharmaceutical industry. The British Minister of Health in May 1963 echoed the pubic’s views cogently as he said “The House and the public suddenly woke up to the fact that any drug manufacturer could market any product however inadequately tested, however dangerous, without having to satisfy any independent body as to its efficacy and safety and the public was almost uniquely unprotected in this respect.” This brought about the Medicines Act 1968. This piece of legislation brought together in a single Act everything to do with control of medicines, for both human and animal use, including promotion and sales. As specified by the Act, all medicines already on the UK market had to go through peer review and be re-approved or withdrawn. The process withered down 32,000 products that originally existed in 1971 leaving only 5000 fully licensed medicines by 1990 when the process was completed (Royal Pharmaceutical Society, 2011). This means 82% of the drugs on the market in 1971 were effectively deemed unsafe and without the newly introduced legislation that required re-evalauation of the drugs, there were potentially danagerous drugs kept on the market.
The thalidomide scandal caused the English and Scottish Standing Medical advisory Committes to recommend future legislation and the immediate formation of the Committees on Safety of Drugs 1963. This committee had no legal power but worked on the basis of voluntary cooperation with the pharmaceutical industry in the UK as a provisional measure until the Medicines Act became law 5 years later in 1968. However, the time from discovery of a new molecule to the launch as a new product is very product dependent (Kirkwood & Longley, 1994).
Today as a follow up from the Act in 1968, regulation strives to achieve the safety of medicines to the highest, safest standards. The MHRA (Medicines and Healthcare Regulations Agency) endeavours to find the right balance beween benefits and risks so as to allow the development of drugs which are initially risky but potentially life-saving, such as cancer remedies, while also avoiding thalidomide-type disasters. It has succeeded on the most part as unlike the USA we have had fewer isolated clinical drug mishaps. However while the drug is being trialled, there have been some unfortunate cases where supposedly safe drugs have had catastrophic and fatal effects. This includes the Paraxel Phase 1 trails also known as TGN1412 trials.
The Paraxel Case refers to the phase 1 clinical study conducted for a CD28 superagonist antibody (a protein) in six healthy human volunteers (Vince, 2006). The incident in 2006 left 6 human volunteers facing life-threathening conditions involving multi-organ failure (Attarwala, 2010). The volunteer worst affected, Ryan Wilson, spent 147 days in hospital with heart, liver and kidney failure. He lost fingers and all his toes were amputated. Mr Wilson’s ‘body tripled in size’ and he had to be put in a medically induced coma (Mail Online, 2008). The drug had been confirmed safe and efficaious in preclinical studies and the volunteers were given 500 times smaller a dose than those found to be safe in animals (Attarwala, 2010). This is evidence that animal testing is yet again inaccurate as this incident highlights that legislation needs to reflect new advances in technology such as epidiemiology and remove the caveat that animal testing be the sole test used to determine efficacy in pre-clinical trials.
The MHRA and the UK Board of Health have done well to avoid any large scale disasters in the UK, on this basis it would seem that the Medicnes Act 1968 has been effective and the mistakes in legislation which contributed to the thalidomide diaster have been learnt from.
Regulations in West Germany
Before thalidomide was launched in October 1957 there was “no consistent body of law regulating pharmaceuticals in the Federal Republic of Germany” (Grunenthal, 2014). Prior to and during the thalidomide tragedy, responsibility lied on pharmaceutical companies to test drugs and prove safety. Even though teratogenicity testing was routine (Hoffman, 1995) ,the law did not require companies to test for it.
The first law to be passed – The West German Drug Law – regarding drug syndication and development was only published in 1961, much too late for a drug that had been marketed since 1957.
After thalidomide, the German parliament drew up the new Act which marked the launch of rigorous procedures for the approval and documentation of new drugs in the Germany. Since then, companies must “present extensive dossiers on the efficacy, safety, and quality of their products to the authorities before any new drug is released for sale” (Kirkwood & Longley, 1994). This has prevented further disasters in West Germany and shows that the German parliament is determined to avoid another thalidomide disaster. The addition of laws to the West Germany legislation has often been hailed “the single most important consequence of the thalidomide disaster” (Grunenthal, 2014) because of the extensive reforms it introduced.
Conclusion
It is impossible to give a unilateral answer as to whether the pharmaceutical and medical industries have “learnt from thalidomide”. In certain aspects such as the swift changes in legislation, spurred by the medical industry, thalidomide and its catastrophic consequences has been learnt from. The Kefauver-Harris Amendment of 1962 has been very effective and has even prevented further disasters with other drugs such as with isotretinoin a prescription drug used to treat severe acne which like thalidomide is a teratogen. The Amendment prompted the FDA and its manufacturer to create a risk management programme which prevented foetal exposure (Tantibanchachai, 2014). Similarly, the Medicines Act 1968 in the UK succeeded in removing unsafe drugs from the market through rigorous assessment criteria. The act succeeded in removing 82% of the drugs on the market in 1971 which were unsafe by 1990 (Royal Pharmaceutical Society, 2011)and has reduced incitement for further disasters today. A well-deserved kudos should be given regarding the swift changes in legislation following thalidomide.
However, the other factors explored in this report, do not show that lessons have been learnt from thalidomide. There is still much to be done in order to control distribution of the drug in South-American countries. The FDA and MHRA have made great progress with the S.T.E.P.S. in the USA and the UK however, this report explores the pharmaceutical industry as a whole. Therefore, with evidence of 100+ cases between 2005-2010 (Crawford, 2013) and 34 cases 1965-1995 (Castilla, et al., 1996), it is conclusive that more needs to be done. The ECLAMC predicts that there is incitement for another thalidomide disaster in coming years (FFDN, 2008) due to the lack of control over distribution despite steps made in western countries. This shows that the world pharmaceutical industry as a whole has not learnt from the physical and mental damages thalidomide caused in Europe and is willing through weak control measures to allow another large-scale disaster to occur in Latin-America. A potential solution that has been suggested by the ECLMC which is viable, is an international body which controls the distribution of thalidomide (Castilla, et al., 1996). Perhaps, this international body can work through the thalidomide trust but the fact is, more needs to be done regarding thalidomide induced embryopathy in South American countries.
The FDA and MHRA in the USA and UK still mandate that animal testing data be used primarily in deciding drug efficacy and safety in phase 1 trials (Medicines and Healthcare Products Regulatory Agency, 2009) ( Animal Friends Croatia, 2001). This has allowed cases such as the Vioxx Scandal – tarred the “worst disaster in drug history” (Drug Watch, 2014) and the disaster of the Paraxel phase 1 trials to occur. Advances in technology seem to render animal testing as the primary test increasingly obsolete, therefore, this report concludes that the failures of animal testing have not been acknowledged by the pharmaceutical industry. The lack of acknowledgement has allowed Paraxel and Vioxx to occur due to faulty animal testing data. This report concludes that the law in partnership with the pharmaceutical industry needs to reflect the new advances in technology such as epidemiology.
Currently, the medical industry has few processes in place to secure off-label prescription even post-thalidomide. Off-label prescription spurred by scientific evidence (such as the use of antidepressants to treat chronic pain) is great and should be encouraged, however, in order to reduce incitement for another thalidomide-type disaster, it is necessary that only scientifically valid and authentic information be distributed by pharmaceutical companies. The use of anecdotal evidence to prescribe a drug off-label should be limited to a case-by-case basis but not on a large-scale use such as with thalidomide. There is little evidence that the medical sector has considered the role off-labelling played in thalidomide and no evidence to suggest that thorough attempts have been made to ensure safety of drugs prescribed to fragile target audiences such as expectant mothers (Gupta & Nayak, 2014). The medical and pharmaceutical sectors have continued with the previous practices that were in use at the time and thalidomide has not been learnt from.
This report concludes that the pharmaceutical industry has learnt from some of the factors that contributed to past mistakes but the over-reliance on animal testing needs to be revaluated and the distribution of thalidomide outside of western countries needs to be controlled with stricter measures.
Reference List
Animal Friends Croatia, 2001. The Tragedy of Thalidomide and the Failure of Animal Testing. [Online] Available at: http://www.prijatelji-zivotinja.hr/index.en.php?id=582 [Accessed 10 February 2017].
Attarwala, H., 2010. TGN1412: From Discovery to Disaster. Journal of Young Pharmacists, 2(3), pp. 332-336.
Bara, F., Athena T, S. & Carias, E., 2009. The Thalidomide Tragedy: Lessons for Drug Safety and Regulation. [Online] Available at: https://helix.northwestern.edu/article/thalidomide-tragedy-lessons-drug-safety-and-regulation [Accessed 28 January 2017].
BBC Panorama, 2004. Why we needed to regulate, London: British Broadcasting Coporation.
Botting, R., 2015. Chapter 18. The History of Thalidomide. In: J. Botting, ed. Animals and Medicine: The Contribution of Animal Experiments to the Control of Disease . Cambridge: Open Book Publishers, pp. 183-199.
British Broadcasting Corporation, 2005. THE RETURN OF THALIDOMIDE. [Online] Available at: http://www.bbc.co.uk/insideout/southwest/series7/thalidomide.shtml [Accessed 2 February 2017].
Castilla, E. et al., 1996. Thalidomide, a current teratogen in South America.. Teratology, 54(6), pp. 273-277.
Crawford, A., 2013. Brazil’s new generation of Thalidomide babies, s.l.: BBC Newsnight.
Department of Curriculum and Instruction, Chinese Unversity of Hong Kong, 2007. Information for Chemistry Teachers. [Online] Available at: http://www3.fed.cuhk.edu.hk/chemistry/files/chiraldrug.pdf [Accessed 27 December 2016].
Drug Watch, 2014. Vioxx Drug Recall Information. [Online] Available at: https://www.drugwatch.com/Vioxx/recall/ [Accessed 7 January 2017].
Evans, I., Thornton, H. & Chalmers, I., 2011. Testing Treatments: Better Research for Better Healthcare. 2nd ed. London: Pinter & Martin.
FFDN, 2008. Children born i Brazil. [Online] Available at: http://www.thalidomide.org/web/children-born-i-brazil/ [Accessed 18 February 2017].
Fulkerson, N., 2011. Testing on animals leads to important medical breakthroughs. [Online] Available at: http://northernstar.info/opinion/columnists/article_94ffd440-60af-11e0-ac68-001a4bcf6878.html [Accessed 18 February 2017].
Greek, R., Shanks, N. & Rice, M. J., 2011. The History and Implications of Testing Thalidomide on Animals. The Journal of Philosophy, Science & Law, 11(3), pp. 1-32.
Grunenthal, 2014. Questions and answers on Contergan and thalidomide. [Online] Available at: http://www.contergan.grunenthal.info/grt-ctg/GRT-CTG/Die_Fakten/FAQ/en_EN/152700081.jsp [Accessed 2 January 2017].
Gupta, S. K. & Nayak, R. P., 2014. Off-label use of medicine: Perspective of physicians, patients, pharmaceutical companies and regulatory authorities. Journal of Pharmacology and Pharmacotherapeutics, 5(2), pp. 88-92.
Hoffman, R., 1995. The Same and Not The Same. 2nd ed. Columbia: Columbia University Press.
Hooper, C. H., 2008. Pharmaceuticals: Economics and Regulation. [Online] Available at: http://www.econlib.org/library/Enc/PharmaceuticalsEconomicsandRegulation.html [Accessed 16 January 2017].
Kirkwood, R. & Longley, A., 1994. Legislative control relating to pharmaceutical chemicals. In: K. A & L. A, eds. Clean Technology and the Environment. s.l.:Springer Science & Business Media, pp. 229-229.
Knapp, V., Christie, G. A. & Seller, M. J., 1962. Thalidomide and Congenital Abnormalities. The Lancet, 280(7249), p. 249.
Lingham, A., 2000. Some Effects of thalidomide. [Online] Available at: http://www.chm.bris.ac.uk/motm/thalidomide/effects.html [Accessed 27 December 2016].
Lo, W. Y. & Friedman, J. M., 2002. Teratogenicity of recently introuduced medications in human pregnancy. Obset Gynecol, 100(3), pp. 465-473.
Mail Online, 2008. ‘Elephant Man’ drug trial victim set to win £2million payout after losing toes and fingers. [Online] Available at: http://www.dailymail.co.uk/news/article-559760/Elephant-Man-drug-trial-victim-set-win-2million-payout-losing-toes-fingers.html [Accessed 19 February 2017].
Medicines and Healthcare Products Regulatory Agency, 2009. Off-label or unlicensed use of medicines: prescribers’ responsibilities. [Online] Available at: https://www.gov.uk/drug-safety-update/off-label-or-unlicensed-use-of-medicines-prescribers-responsibilities [Accessed 2 January 2017].
Miller, K., 2015. Off-Label Drug Use: What You Need to Know. [Online] Available at: http://www.webmd.com/a-to-z-guides/features/off-label-drug-use-what-you-need-to-know#1 [Accessed 2 January 2017].
Miller, M. T., 1991. “Thalidomide Embryopathy: A Model for the Study of Congenital Incomitant Horizontal Strabismus”.. Miller, Marylin T. (1991). “Thalidomide Embryopathy: A Model for the Study The American Ophthalmological Society.
Miller, M. T. & Stromland, K., 1991. Teratogen Update: Thalidomide A Review, With a Focus on Ocular Findings and New Potential Uses. Trans Am Ophthalmol Soc, Volume LXXXIX.
Pannikar, D. V., 2003. The Return of Thalidomide: New Uses and Renewed Concerns, s.l.: WHO.
Paumgartten, F. & Chahoud, I., 2006. Thalidomide embryopathy cases in Brazil after 1965. Reproductive Toxicology, 22(1), pp. 1-2.
Pippin, D. J., 2005. The Need for Revision of Pre-Market Testing ; The Failure of Animal Tests of COX-2 Inhibitors, Washington: Arthritis Advisory Committee & Drug Safety and Risk Management Advisory Committee.
Royal Pharmaceutical Society, 2011. The evolution of pharmacy. [Online] Available at: www.rpharms.com/learning-resources/the-evolution-of-pharmacy.asp [Accessed 19 February 2017].
Ryder, S.-A. & Stannard, C. F., 2005. Treatment of chronic pain: antidepressant, antiepileptic and antiarrhythmic drugs. Continuing Education in Anaesthesia, Critical Care & Pain, 1 February, 5(1), pp. 18-21.
Safer Medicines, 2015. Could the thalidomide tragedy have been averted by more extensive animal testing?. [Online] Available at: http://www.safermedicines.org/page/faqs_faq17 [Accessed 2 January 2017].
Schardein, J., 1976. Drugs as teratogens. 2nd ed. Florida: CRC Ppress.
Seller, M. J., 1962. Thalidomide and Congenital Abnormalities. The Lancet, 7249(280), pp. 249-249.
Silverman, W., 2002. The schizophrenic career of a ‘monster drug’. Pediatrics, 110(2), pp. 404-406.
Stephens, T. & Rock, B., 2001. The Dark Remedy: The Impact of Thalidomide and Its Revival as a Vital Medicine. 1 ed. s.l.:Perseus Publishing.
Tantibanchachai, C., 2014. US Regulatory Response to Thalidomide (1950-2000). [Online] Available at: http://hdl.handle.net/10776/7733 [Accessed 18 February 2017].
Taussig, H., 1962. A Study of the German Outbreak of Phocomelia: The Thalidomide Syndrome. Journal of the American Medical Association, Volume 180, pp. 1106-1114.
Teeling-Smith, P. G., 1980. A Question of Balance; the benefits and risks ofo pharmaceutical innovation. London: Office of Health Economics.
Thompson, F., 2016. How U.S. regulation prevented off-label use of a drug and a subsequent birth defect disaster. [Online] Available at: https://www.motleyrice.com/blogpost/us-regulation-prevented-off-label-drug-birth-defect-disaster [Accessed 31 January 2017].
Vince, G., 2006. UK drug trial disaster – the official report. [Online] Available at: https://www.newscientist.com/article/dn9226-uk-drug-trial-disaster-the-official-report/amp/ [Accessed 19 February 2017].
Warren, R., 1999. The Many Faces of Thalidomide ( from 1957 to 1966). [Online] Available at: www.thalidomide.ca/many-faces-of-thalidomidde/ [Accessed 14 February 2017].
Bibliography
Chhabra, N., Aseri, M. L. & Padmanabhan, D., 2013. A review of drug isomerism and its importance. International Journal of Applied and Basic Medical Research, 3(1), pp. 16-18.
Evans, H., 2014. Thalidomide: how men who blighter lives of thousands evaded justice. [Online] Available at: https://www.theguardian.com/society/2014/nov/14/-sp-thalidomide-pill-how-evaded-justice [Accessed 3 January 2017].
Hong Kong Education Limited, n.d. Chiral Drugs. [Online] Available at: http://cd1.edb.hkedcity.net/cd/science/chemistry/s67chem/pdf/sRL_4_Chiral_drugs.pdf [Accessed 27 December 2016].
Iyer, C. G. S. et al., 1971. WHO coordinated short term double blind trial with thalidomide on the treatment of acute lepra reactons in male lepromatous cases. Bull World Health Org, 45(6), pp. 719-732.
Keller, S. J. & Smith, K. M., 1982. Animal Virus screen for potential teratogens. I. Poxvirus morphogenesis. Teratogenesis, Carcinogenesis, and Mutagenesis, 2(4), pp. 361-374.
Lux, F., 2010. Thalidomide: uncovering the mystery behind the disaster. [Online] Available at: http://newsciencejournalism.com/03/2010/thalidomide-uncovering-the-mystery-behind-the-disaster/ [Accessed 29 December 2016].
Nguyen, L. A., He, H. & Pham-Huy, C., 2006. Chiral Drug: An Overview. International Journal of Biomedical Science, June, 2(2), pp. 85-100.
Schmid, D. B., 1987. Trends in Pharmacological Sciences, Volume 8, p. 133.
Staples, R. E. & Holtkamp, D. E., 1963. Effects of Parental Thalidomide Treatment on Gestation and Fetal Development. Exp Mol Pathol Suppl, 2(12), pp. 81-106.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Pharmacology"
Pharmacology involves the study of drugs and how they affect the body. A pharmacologist contributes to drug development by researching and testing how the body reacts to medication, and whether the medication can have a positive impact on the body in terms of fighting illness and disease.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




