Efficiency of Protraction Facemask with Alternate Rapid Maxillary Expansion and Constriction: A Systematic Review of Randomised Trials
Info: 10758 words (43 pages) Dissertation
Published: 16th Dec 2019
Tagged: Dentistry
The efficiency of protraction facemask with alternate rapid maxillary expansion and constriction versus rapid maxillary expansion in correction of Class III malocclusion in children: A systematic review of randomised trials
Abstract:
Background:
Class III correction for maxillary deficiency in children and adolescents has been carried out using protraction facemask (FM) in conjunction with rapid maxillary expansion (RME) or rapid maxillary expansion and constriction (Alt-RAMEC). The aim of this systematic review was to evaluate the difference in effect between RME and Alt-RAMEC when used with protraction FM for the correction of maxillary deficiency in class III malocclusion for children and adolescents. The primary outcomes assessed were overjet correction, and linear or angular skeletal changes.
Methods:
Data sources were electronic databases including MEDLINE, EMBASE, Scopus, Cochrane Central Register of Controlled Trials up to January 2017. No language restrictions were applied. Search terms included ‘Class III malocclusion’ and ‘orthopaedic treatment’. Randomised clinical trials (RCT) comparing protraction FM in conjunction with either RME or Alt-RAMEC were suitable for inclusion. Articles were excluded if functional or multi-bracket appliances had been used, or if patients had craniofacial syndromes. Two authors undertook independent data extraction using predefined forms. Disagreements were resolved by discussion and consultation with the third author. The Cochrane risk of bias tool was used to assess the quality of the studies.
Results:
Only one study met the inclusion criteria. 44 patients were included in this study (22 in Alt-RAMEC/FM and 22 in RME/FM group). The risk of bias was unclear. Correction of overjet was not reported. Maxillary forward movement increased by 3.04 mm in the RPE/C group, which was significantly greater than that in the RPE group (2.11 mm) (ES, -0.93 [95% CI, -1.63, -0.23; P = 0.013]). These differences may not be clinically important.
Conclusions:
There is weak evidence suggesting that protraction FM with Alt-RAMEC may result in more maxillary forward movement than FM with RME. Higher quality RCTs are required to inform clinical practice.
Funding:
There was no funding received for this review.
Registration:
PROSPERO International prospective register of systematic reviews database ID No. 42017069410
Key words: human study; malocclusion; Class III; systematic review; protraction FM; review article
BACKGROUND
Description of the condition
The forward position of the mandible or posterior position of the maxilla or both ( reverse overjet, reverse bite; under bite; Class III malocclusion) may be due to genetic or environmental factors or both affecting jaws and teeth size and positions. In case of forward position of the mandible, treatment philosophy had been directed to preventing further growth of the mandible by using a chin cup. Studies has shown that treatment effect was rather a redirection of the mandibular growth in a downward and backward direction (Graber, 1977; Ritucci et al., 1986; Suguwara et al., 1990), rather than due to a true orthopedic effect.
In case of maxillary backward position (maxillary deficiency), early treatment (Baccetti et al. 1998, Mandall et al. 2016) has been attempted with a combination of chin cup and maxillary protraction device (FM) to redirect maxillary growth in a forward and downward direction with an accompanying backward rotation of the mandible (Kim et al. 1999, Cordasco et al. 2014). A recent systematic review showed some evidence for the effectiveness of the FM appliance in the short term, but reported no evidence that the results are maintained in the long term (Watkinson et al. 2013). However, more recently a long term randomized controlled trial (RCT) concluded that early class III protraction FM treatment reduces the need for orthognathic surgery from two-thirds for the control group to one-third for the FM group (Mandall et al. 2016).
Previous reports showed improvement of maxillary position, in cases with maxillary deficiency, with protraction FM without concomitant use of RME (Kiliçoglu and Kirliç 1998, Showkatbakhsh et al. 2013). However it is common for RME to be carried out before protraction FM to allow for expansion of a narrow maxilla and potentially enhance the anteroposterior correction by loosening of the circummaxillary sutures (McNamara Jr 1987, Turley 2007).
A technique of alternate rapid maxillary expansion and constriction (Alt-RAMEC or Alt-RAMEC) was introduced, more than 10 years ago (Liou 2005, Liou 2005), aiming to simulate the distraction osteogenesis technique through what the author called sutural expansion/ protraction osteogenesis. This technique was suggested to have greater disarticulation at the circummaxillary sutures; which was supported by an animal study (Wang et al. 2009).
However, the results of studies that utilized the Alt-RAMEC technique are conflicting. Liou and Tsai (2005) used Alt-RAMEC in a controlled clinical trial (1 mm per day for 9 weeks), followed by maxillary protraction, with an intraoral device in 10 patients with clefts. The study demonstrated superior achievement of maxillary forward movement in the Alt-RAMEC group relative to the comparison group of cleft patients whose maxillary protraction was preceded by expansion alone. Similar result was shown by Isci et al. (2010) where the repetitive activation and deactivation protocol resulted in more effective protraction of the maxilla than in the RME alone and again similar results were found in a controlled study (Masucci et al. 2014). On the other hand da Luz Vieira et al. (2009) found no significant difference between 2 groups of 10 patients with cleft lip and palate who were treated with FM maxillary protraction after receiving either Alt-RAMEC, or solely RME with a modified Haas-type palatal expander.
Furthermore, some authors showed that early FM therapy, with or without palatal expansion, was effective to correct skeletal Class III malocclusions (Vaughn et al. 2005, Yavuz et al. 2012) and advocated RME be based on clinical criteria other than assisting the Class III correction. RME before using FM is still common practice, but less so for Alt-RAMEC. However, there is no consensus on the best expansion method for use with FM.
OBJECTIVES
To assess the difference in treatment effectiveness of protraction FM with RME compared with FM with Alt-RAMEC children between 7-14 years of age.
METHODS
Protocol and registration
This systematic review protocol was registered with the International prospective register of systematic reviews (PROSPERO) database (ID No. 42017069410). Available from http://www.crd.york.ac.uk/PROSPERO/displayrecord.asp?ID=CRD42017069410.
Eligibility Criteria
The following eligibility criteria were applied (PICOS):
(1) Population: subjects with Class III malocclusion between 5 and 14 years of age (children or adolescents or both);
(2) Intervention: FM with Alt-RAMEC;
(3) Comparison: FM with RME;
4) Outcome;
Primary outcomes:
- Correction of reverse overjet (measured in millimetres or by other index of malocclusion) with the measurements based on study models, or cephalometric or clinical assessment.
- Linear and angular skeletal changes
Secondary outcomes:
- Patient perception.
- Oral health related quality of life.
- Long term effects.
- The reduction in need of orthognathic surgery following treatment.
(5) Types of studies: Only RCTs were considered
Studies including patients with an orofacial clefting, syndromes, or who had previously received surgical treatment aiming at correction of the Class III malocclusion were excluded. Also, studies were excluded if additional and concomitant treatment were carried out (e.g. fixed appliances, extractions,etc.) to avoid confounding treatment effects.
Search methods and studies selection
The following databases were searched for relevant studies: The Cochrane Central Register of Controlled Trials, Pubmed (from 1946 onwards), Embase Classic+Embase (from 1947 onwards), Ovid (from 1947 onwards), and Scopus (from 1960 onwards) were searched on 30 January 2017. Search terms were ‘Class III malocclusion’ and ‘orthopaedic treatment’. The search strategy for PubMed is provided in Table 1. No restrictions were placed on the language or date of publication when searching the electronic databases. We searched the reference lists of included studies for any relevant RCTs.
Table 1 PubMed search strategy
| #1 Randomised controlled trial |
| #2 Prospective study |
| # 3 Clinical study |
| #4 1 OR #2 OR #3 |
| #5 Orthopedic treatment Class III |
| #6 #4 AND #5 AND Humans[Filter] |
Data collection and analysis
Study selection
Studies were screened for duplicates using the “find duplicates” function in Endnote version X8TM (Clarivate Analytics, Philadelphia, USA) and through a manual search. Two authors (AA and AU) were responsible for screening the studies by title and abstract independently and in duplicate.
Full text of potentially eligible study/studies were retrieved and checked for compliance with the eligibility criteria. This was performed by two review authors (AU and AA) independently and in duplicate. Disagreements at the screening and inclusion stages were resolved by discussion and consultation with the third author (Bodore Albaker (BA)). An attempt to contact the original study authors was intended for clarification of study eligibility or additional information if required. A study was not planned to be excluded from this review because of missing summary data, however the potential implications of their absence from any meta-analysis were planned to be discussed.
Articles not in English were assessed by their abstract, where possible. We planned to translate the full text if they appeared to be eligible for inclusion in the study.
Data extraction
A customized data collection form was created and used to gather information from the selected studies. This information included:
- authors, year of publication, language; demographic details of the report;
- details of the interventions, inclusion and exclusion criteria; appliance’s features;
- type of trial, sample size, age, gender, method of randomisation, allocation concealment, blinding, method of assessing the outcomes and drop-outs;
- time of treatment and observation, time of daily appliance wear, presence of follow-up characteristics of participants, outcome measures and results of the intervention.
The data extraction was performed by both two authors independently and in duplication. Disagreements were resolved by discussion and consultation with the third author.
The following linear and angular cephalometric parameters were collected as outcomes: A point to vertical axis, SNA, SNB, ANB, SN-palatal plane, and SN-mandibular plane.
Risk of bias and quality assessment in the included studies
The risk of bias for the RCTs was evaluated using the Cochrane Collaboration’s tool for assessing the risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.2.0 (Higgins et al. 2017). This was undertaken independently and in duplicate by two review authors (AU, AA) as a part of the data extraction process. Six specific domains were investigated: Random sequence generation (selection bias); allocation concealment (selection bias); blinding of outcome assessors (detection bias); incomplete outcome data (attrition bias); selective outcome reporting (reporting bias); and ’other sources of bias’. Each domain was given a judgement that could be high, low, or unclear. ’High’ indicated a high risk of bias, ’Low’ a low risk of bias, and ’Unclear’ indicated an unclear or unknown level of bias. We did not evaluate blinding of operator and participant as blinding to the intervention was unfeasible in most circumstances.
Following assessment of each domain, we assessed the overall risk of bias for each study. All domains contributed equally to the overall study risk of bias: we considered a study at high risk of bias when at least one domain was judged as high risk of bias; we considered studies with at least one unclear domain at an unclear risk of bias; we considered studies with all risk of bias domains judged as low as low risk of bias (Higgins et al. 2017).
Measures of treatment effect
Data for the outcomes in the included study were continuous. Mean differences and standard deviations were used to summarise the data for each group where the mean differences and 95% confidence intervals were calculable from the data presented.
We did not conduct a meta-analysis, as only one study was eligible to be included in this review. For measures of treatment effects that will be used in future updates of this review; The Cochrane Collaboration statistical guidelines will be followed and the data will be analysed and reported according to Cochrane Collaboration criteria. For continuous outcomes, mean differences and 95% confidence intervals would be used to summarise the data for each group where the mean differences and standard deviations are calculable from the data presented. For dichotomous data, the estimates of the effect of an intervention would be expressed as risk ratios together with 95% confidence intervals.
Dealing with missing data
We reported on the levels of loss to follow-up and assessed this as a source of potential bias.
Attempts were made to contact trialists to obtain missing data but these were unavailable to us. In future updates if data are missing from trials, reasonable attempts will be made to contact the investigators or sponsors of these studies. We will re-analyse data according to the intention-to-treat (ITT) principle whenever possible. For dichotomous outcomes, if authors had conducted a per-protocol analysis we will carry out an ITT analysis with imputation setting the missing data to their baseline values, after checking the degree of imbalance in the drop-outs between the arms to determine the potential impact of bias (section 16.2.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)). For continuous outcomes a per-protocol analysis will be carried out in place of an ITT analysis.
Assessment of heterogeneity
With sufficient studies, clinical heterogeneity would be assessed by examining the characteristics of the included studies: the differences between the types of participants, the interventions and the outcomes within and across the trials. Statistical homogeneity would be assessed using X2 test in addition to the I2 statistic as follows
If there was considerable heterogeneity (I² > 50%), which could not be explained by the subgroup analyses, we planned not to conduct meta-analysis. We had planned to interpret I² values between 0% to 40% as possibly insignificant, 30% to 60% as possibly significant, 50% to 90% as possibly substantial, and 75% to 100% as possibly very substantial (‘considerable’); depending on whether the inconsistency in results was due to differences in the direction of effect estimates between trials rather than due to differences in the magnitude of effect estimates favouring an intervention (Deeks et al. 2017). This method would be used for future updates of this review.
Assessment of reporting biases
If there were more than 10 studies for one outcome we planned to construct a funnel plot. If there was asymmetry in the funnel plot, indicating possible publication bias, we planned to undertake statistical analysis using the methods introduced by (Egger et al. 1997) (continuous outcome) and (Rücker et al. 2008) (dichotomous outcome). Insufficient studies were identified to investigate reporting biases.
Data synthesis
We analysed the data using Review Manager 5 software (REVMAN 2014). We did not conduct a meta-analysis, as we included only one study in this review. Instead, we presented the main study’s results with a narrative description, summary table and forest plots.
If sufficient studies were found, we planned to use the fixed-effect and random-effects models as appropriate, for the synthesis and meta-analysis of any quantitative data. If we establish that there is heterogeneity between the studies, we may undertake a random-effects model as appropriate, but if the heterogeneity between the studies is significant, we may not undertake a meta-analysis. If there are too few clinically homogenous trials or insufficient data for pooling, we will present the results of the individual trials and perform a descriptive analysis only.
Subgroup analysis and investigation of heterogeneity
No subgroup analyses were planned.
Sensitivity analysis
Providing there were sufficient studies for each intervention and outcome, sensitivity analyses were planned in order to examine the effect of the quality assessment items on the overall estimates of effect (including low risk of bias studies only).
Presentation of main results
We used the GRADE approach to describe the quality of the evidence (Schünemann et al. 2017). We used the Guideline Development Tool (GRADEpro GDT 2015)to construct ‘Summary of findings’ tables for each comparison included in the review.
The quality of the body of evidence was assessed with reference to the overall risk of bias of the included studies; the directness of the evidence; the consistency of the results; the precision of the estimates; the risk of publication bias; and the magnitude of the effect. The quality of the body of evidence for each of the primary outcomes was categorised as high, moderate, low or very low, and summary of findings tables have been produced for the main outcomes of this review.
We initially gave evidence from the RCT a rating of moderate quality. However, we downgraded this rating on the basis of specific criteria: Unclear risk of bias, small sample size; wide confidence intervals (CIs) for the estimates of effects; failure to adhere to intention-to-treat analysis (Ryan and Hill 2016). Thus, it was possible for data from RCTs to be given a low-quality rating if several of these concerns were present.
RESULTS
Study selection and characteristics
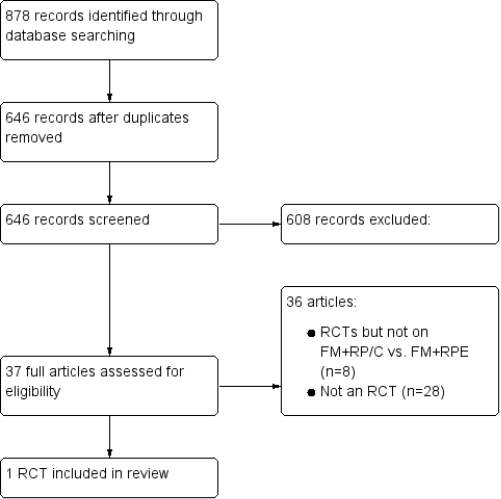
A total of 878 citations were obtained after data search. After exclusion of duplicate reports, 646 remained. Of these, 524 studies were excluded by title while 84 were excluded by abstract, as it appeared that these papers clearly did not meet the inclusion criteria.
Figure 2 illustrates the flow diagram of the selection of the studies process according to guidelines provided by the PRISMA statement (Liberati et al. 2009).
From the records that were identified, 37 full-text articles were retrieved for further evaluation in more detail. Thirty-six articles (of which 8 were RCTs) were subsequently excluded with reasons for exclusion of exclusion of RCTs shown in (Table 3). Only 1 RCT (Liu et al. 2015) was identified as eligible to be included in this analysis. No unpublished relevant studies were obtained. The characteristics of the included study are shown in Table 5.
| Liu et al 2015
|
|
| Methods | 2-arm parallel randomised controlled trial |
| Participants | 44 patients:2 groups:
22 to RPE and 22 to Alt-RAMEC. 1 patient lost to follow up in RME Final sample (n=43): RPE=10 males, 11 females; Alt-RAMEC = 10 males, 12 females Age: RME: 9.81 (1.72, SD), Alt-RAMEC: 10.11 (1.44, SD) Inclusion criteria: Age 7-13 years with midface soft tissue deficiency. Fully erupted maxillary first molars. Patients had Skeletal Class III malocclusion (ANB<0°), Anterior crossbite, Wits appraisal less than 2 mm, distance from Point A to nasion perpendicular less than 0 mm (corrected cephalometric tracing technique was applied for patients with functional shift), Exclusion criteria: 1. Previous orthodontic treatment 2. Other craniofacial anomalies, such as cleft lip and palate 3. Maxillary dentition unsuitable to bond a hyrax expander Setting: Patients were recruited from the Department of Orthodontics, Peking University, Beijing, China |
| Intervention | Comparison between FM protraction combined with Alt-RAMEC vs RME alone. Patients divided into 2 groups
Group 1: treated with RME for 1 week (4 turns= 1 mm per day) followed by FM maxillary protraction, delivering force of 400-500 g per side Group 2: treated with Alt-RAMEC for 7 weeks ( The sequence was 7 days of expansion, 7 days of constriction, 7 days of expansion, 7 days of constriction, 7 days of expansion, 7 days of constriction, and then a final 7 days of expansion) followed by FM maxillary protraction, delivering force of 400-500 g per side In both groups, direction of force was 15° to 30° downward from the occlusal plane. The patients were instructed to wear the FM for at least 14 hours a day |
| Outcomes | Skeletal changes
All measurement taken before treatment and when positive overjet with Class I or Class II molars were achieved No retention appliances were used after treatment. (( Primary: degree of maxillary forward movement after treatment Secondary: changes of the other cephalometric variables after treatment and the treatment time)) |
| Notes | Sample size calculation was estimated usingG*Power (version 3.0.8) using a previous study on 2-hinged expander Alt-RAMEC and intraoral maxillary protraction (95% power; 5% significance level; 2-tailed); minimum sample size of 16 in each group required to detect significant difference in ANS between groups; sample size was increased by 40% to account for dropouts, resulting in 22 patents in each group |
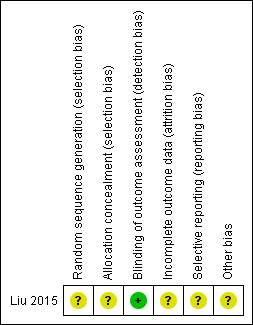
Risk of bias in included studies
The quality assessments of the RCT included in this review are given in REF _Ref489384957 h * MERGEFORMAT Table 3Table 3 and Figure 3 .
All domains were judged as having unclear risk of bias except detection bias assessment which was judged as having low risk of bias
The Overall risk of bias was assessed as unclear.
| Bias | Author’s Judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was carried out using a random number table from a medical statistics textbook, However, the process not supervised |
| Allocation concealment (selection bias) | Unclear risk | Forty-four envelopes containing the subjects’ information were used to ensure allocation concealment from the researchers. Sequential numbers were written on the envelopes.
One investigator was responsible for generating and implementing the random allocation process, enrolling participants, and opening the envelopes in sequence, However, the process was not supervised. |
| Blinding (performance bias and detection bias)
All outcomes |
ULow risk | Blinding of the assessor was carried out during the cephalometric analysis. All cephalometric films were deidentified by opaque tape and replaced by research numbers, and then disarranged before tracing. The investigator who was responsible for measuring did not know the grouping of the cephalometric radiographs. |
| Incomplete outcome data (attrition bias)
All outcomes |
Unclear risk | Low withdrawal rates (1/44). However, the One subject in the RME group declined to have the final x-rays and was excluded from the analysis. No intention-to-treat analysis carried out. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable. Unclear whether outcomes were reported as per trial registration. Overjet not reported although a positive overjet indicated treatment completion. Starting overjet could have been a confounding factor in treatment duration and other secondary outcomes |
| Other bias | Unclear risk | None detectable. The authors reported that patients satisfaction with treatment was similar in the RME and Alt-RAMEC groups. However, it was not clear how the authors arrived at this conclusion. |
| Overall risk of bias: | Unclear risk |


Effects of interventions
For the current version of this review, the only included trial (Liu et al. 2015) consisted of 43 children with Class III malocclusion who were treated for 10.95 (±2.73) months for the FM+ Alt-RAMEC group and 11.19 (±2.75) months for the FM+RME group. As the review includes only one trial, we were unable to conduct a meta-analysis for our two primary outcomes (correction of reverse overjet and linear and angular skeletal changes) and other secondary outcomes. We did not assess heterogeneity and we did not perform subgroup analyses or sensitivity analyses. Below, we provide a narrative description of the results of the included study (Table 4).
Table 4: Summary of findings for the main comparison
| FM+Alt-RAMEC compared with FM+RME for Class III malocclusion | |||||||
| Patient or population: Children and adolescents with Class III malocclusion
Settings: Dental Hospital Intervention: FM+Alt-RAMEC Comparison: FM+RME |
|||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
No of Participants (studies) |
Quality of the evidence (GRADE) |
Comments | ||
| Assumed risk | Corresponding risk | ||||||
| [control] | [experimental] | ||||||
| Overjet change | See comment | See comment | – | See comment | No study addressed this outcome | ||
| A point to vertical axis change in mm (A-VRL) | The mean forward A point movement in the control groups was 2.11 mm ±0.95 | The mean forward A point movement in the intervention groups was 0.93 mm higher (0.23 to 1.63 higher) | 43 (1) |
⊕⊕⊕⊝ moderate |
|||
| SNA angle change in degrees
|
The mean change in SNA angle in the control group was 1.93° ±0.79 | The mean change in SNA angle in the intervention group was 0.77° higher (10.13 to 1.41 higher) | 43 (1) |
⊕⊕⊕⊝ moderate |
|||
| SNB angle change in degrees
|
The mean change in SNB angle in the control group was -2.35° ±1.21 | The mean change in SNB angle in the intervention group was 0.86° lower (0.22 to 1.50 lower) | |||||
| Palatal plane change to SN line in degrees
SN/PP
|
The mean counterclockwise rotation of the palatal plane in the control group was 0.83° ±1.17 | The mean counterclockwise rotation of the palatal plane in the intervention group was 0.90° higher (0.10 to 1.70 higher). | 43 (1) |
⊕⊕⊕⊝ moderate |
|||
| Mandibular plane change to SN line in degrees
MP/SN |
The mean clockwise rotation in the intervention groups was 3.32° ±1.91 | The mean clockwise rotation in the intervention groups was 1.32 lower (0.27 to 2.37 lower) |
43 (1) |
⊕⊕⊕⊝ moderate |
|||
| Protraction time in months | The average protraction time (from the beginning to the end of protraction) was 10.84 ± 2.76 months in the control group | The average protraction time was 1.78 months lower [3.34 to 0.22 lower] | 43 (1) |
⊕⊕⊕⊝ moderate |
|||
| Total treatment time in months | The average total treatment times (from the beginning
of expansion to the end of protraction) was 11.19 ±2.75 months in control group |
The average total treatment time was 0.24 months lower [1.88 lower to 1.40 higher] | 43 (1) |
⊕⊕⊕⊝ moderate |
|||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; [other abbreviations, eg. OR, etc] GRADE Working Group grades of evidence |
|||||||
Primary outcomes
The included RCT fulfilled the inclusion criteria of this review as they set achievement of a positive overjet with a Class II or a Class I molar relationship as the treatment completion criterion.
The primary outcome of the included study was degree of maxillary forward movement after treatment A point to vertical axis (A-VRL).
Overjet change:
Overjet was not reported as an outcome. No information was provided on the pretreatment overjet, post treatment overjet and overjet changes for any of the two study groups
Skeletal changes:
The study also assessed some angular and linear skeletal changes which are presented below.
Movement of A point to vertical line (A-VRL):
There was a 3.04 mm mean forward movement of the A point in relation to the vertical axis in the FM+Alt-RAMEC group which was significantly greater than that in the FM+RME group (2.11 mm), Effect size (ES): -0.93 (95% CI:-1.63-0.23; P =0.009).
SNA change:
The SNA angle significantly increased in the FM+Alt-RAMEC group compared to the FM+RME group, being 2.67°±1.31 and 1.93° ±0.79 , respectively, ES:-0.77 (95% CI: -1.41, -0.13; P = 0.02);
SNB change:
The SNB angle mean reduction was 2.35° ±1.21 in the FM+RME group which was statisticaly significant that the mean reduction of 1.49° ±0.89 in the FM+Alt-RAMEC group (ES=-0.86, (95% CI:-1.50, -0.22; P 5 0.008 )
SN to palatal plane angle:
There was more and significant counterclockwise rotation of the palatal plane of 1.73° ±1.48 in the Alt-RAMEC group, than in the RME group (0.83° ±1.17) (ES= 0.90 [95% CI, 0.10, 1.70; P= 0.03).
SN to mandibular plane angle
The degree of mandibular downward and backward rotation was significantly smaller in the Alt-RAMEC group (2.00° ±1.59) compared to 3.32° ±1.91, ES: 1.32 (95% CI: 0.27, 2.37; P = 0.01)
Secondary outcomes
Treatment time was not included in our study protocol, but was added as a secondary outcome due to the importance of this factor in treatment decsions.
Protraction time in months
The average protraction time (from the beginning to the end of protraction) was 10.84 ± 2.76 months in the FM+RME group, which was significantly longer than in the FM+Alt-RAMEC group (9.06 ±2.55 months) (ES, 1.78 [95% CI, 0.22, 3.34; P 5 0.03).
Total treatment time in months
The average total treatment times (from the beginning of expansion to the end of protraction) were 11.19 ±2.75 months in the FM+RME group and 10.95 ±2.73 months in the FM+Alt-RAMEC group. No significant difference was found between the groups (effect size [ES], 0.24 [95% CI, _1.40, 1.88; P 5 0.77).
Forest plots for the study results
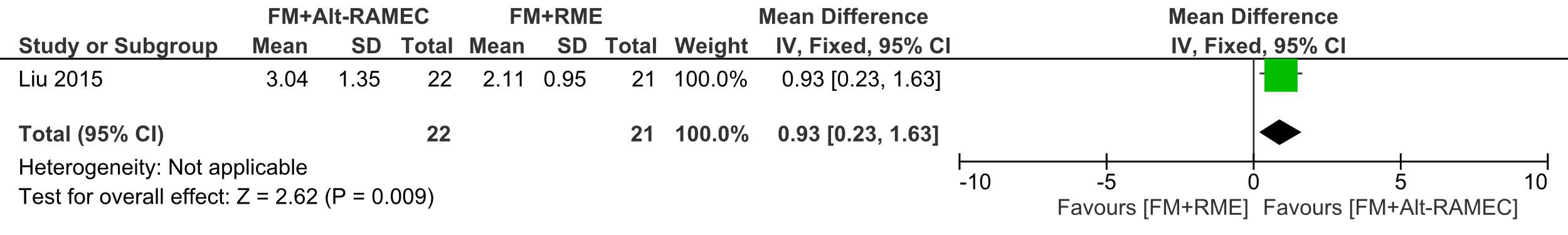 Forest plot of comparison 1: Comparison between FM+RME/C versus FM+Alt-RAMEC, outcome: 1.1 A point to vertical axis change in mm.
Forest plot of comparison 1: Comparison between FM+RME/C versus FM+Alt-RAMEC, outcome: 1.1 A point to vertical axis change in mm.
Forest plot of comparison 2: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.2 SNA angle change in degrees.
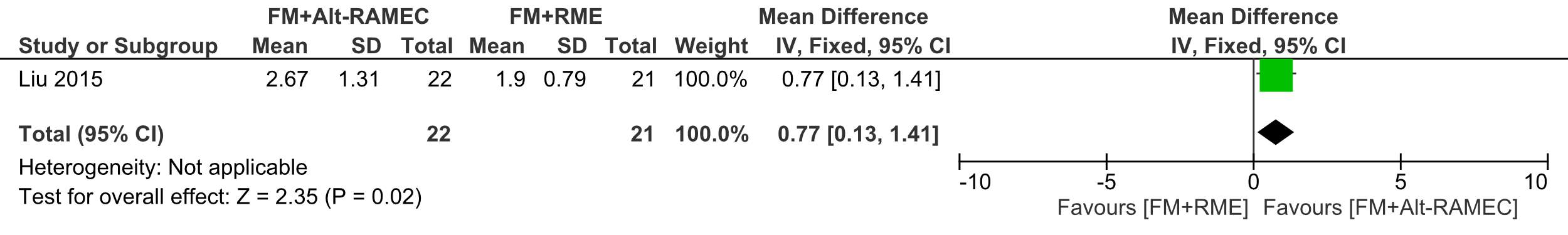
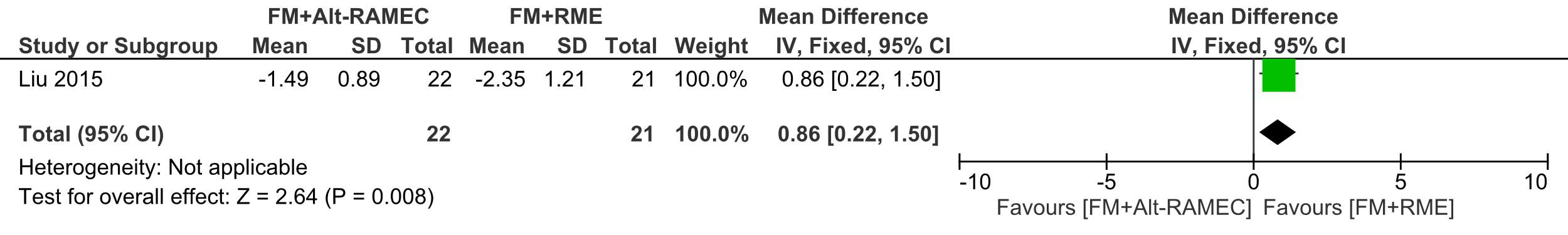 Forest plot of comparison 3: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.3 SNB angle change in degrees.
Forest plot of comparison 3: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.3 SNB angle change in degrees.
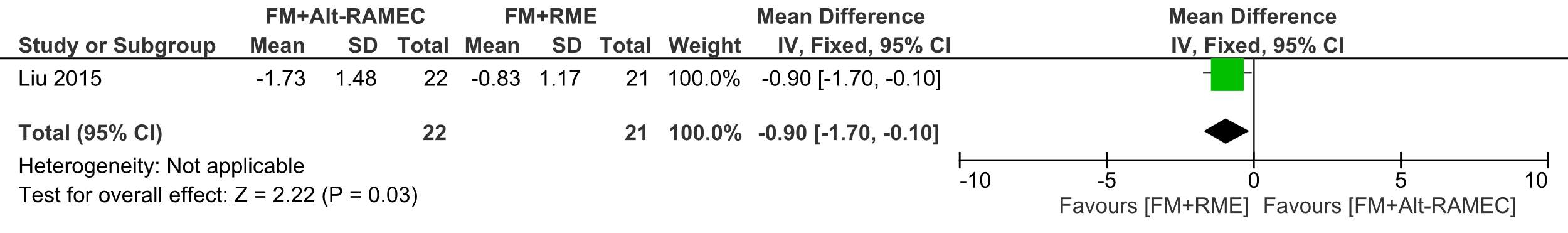 Forest plot of comparison 4: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.4 SN line to palatal plane.
Forest plot of comparison 4: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.4 SN line to palatal plane.
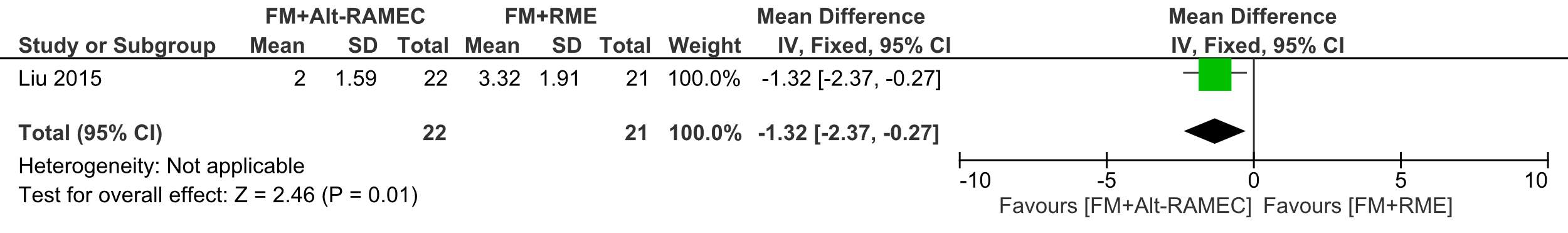 Forest plot of comparison 5: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.5 SN to mandibular plane angle.
Forest plot of comparison 5: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.5 SN to mandibular plane angle.
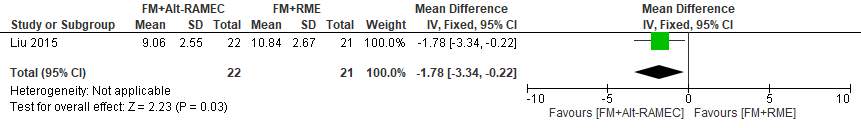 Forest plot of comparison 6: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.8 Average protraction time in months
Forest plot of comparison 6: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.8 Average protraction time in months
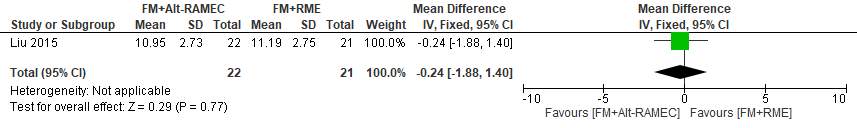 Forest plot of comparison 7: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.9 Average total treatment time in months.
Forest plot of comparison 7: Comparison between FM+RME/C versus FM+RME/C, outcome: 1.9 Average total treatment time in months.
Reliability assessment in the included RCT study
One investigator was responsible for reliability assessment for cephalometric radiograph measurements. Intra-examiner assessment was reported used Dahlberg’s formula.
Strength of evidence
The single included RCT was rated as providing moderate evidence, but we downgraded the overall strength of evidence by one level to low because of the unclear risk of bias, small study sample size (only 43 participants), wide confidence intervals for the estimates of effects and failure to adhere to intention-to-treat analysis. Uncertainty persists regarding the true effect.
Discussion
Summary of main results
In this systematic review, despite a wide and accurate bibliographic search strategy we found only one eligible RCT which has compared the effectiveness of FM with rapid maxillary expansion versus rapid maxillary expansion and constriction. A meta-analysis therefore could not be done for that reason.
Liu et al (2015) was the included study and despite it showing some evidence that favoured the intervention, it should be interpreted with caution because of the small sample size (n=43), unclear risk of bias in most of the assessed aspects. Therefore, no robust evidence for the difference of effect of FM with either Alt-RAMEC or RME.
Overall completeness and applicability of evidence
As mentioned above, one trial consisting of 43 children is not sufficient to provide robust evidence for a difference in effectiveness between FM with either RME or Alt-RAMEC. All of the included participants were children and adolescents (participants’ ages ranged from seven to thirteen years), and there were 20 males and 23 females. The total treatment duration was 11.19 ±2.75 months in the RME group and 10.95 ±2.73 months in the Alt-RAMEC group, therefore these findings are for short term and confined only to that age group.
We were unable to conduct a meta-analysis for our two primary outcomes (correction of reverse overjet and linear and angular skeletal changes) and other secondary outcomes because only one study was included. We could draw no robust conclusions.
Upon considering all available evidence, we found no high-quality evidence for the benefits of FM with Alt-RAMEC over FM with RME. Thus, future clinical studies might be required to assess a true difference between the 2 treatment modalities.
Quality of the evidence
Risk of bias was generally unclear in the domains assessed in the included trial, and overall it was judges as having an unclear risk of bias. Selection bias may exist in this study because the lack of supervision in the randomisation and allocation process. There was a low withdrawal rate in this study (1/44), however, the dropped-out case was excluded from the final analysis giving unclear attrition bias risk. It might be argued that the proportion of missing outcomes was not enough to have a clinically relevant impact on the intervention effect estimate. However, lack of intention to treat analysis was a shortcoming. We considered detection biases to have low risk because the study achieved and reported blinding of the outcome assessors to groups assignments. The reporting bias was judged to be unclear because of the lack of the study protocol.
Using the GRADE approach (see Summary of findings for the main comparison), we rated the quality of this body of evidence as moderate, but we downgraded the overall strength of evidence by one level to low because unclear risk of bias, small study sample size (only 43 participants), wide confidence intervals (CIs) for the estimates of effects and failure to adhere to intention-to-treat analysis. In the presence of one trial uncertainty persists regarding the true effect. The results do not allow us to draw any firm conclusions regarding whether one of these interventions is more effective than the other.
Potential biases in the review process
Although we attempted to minimize publication bias by using a comprehensive search strategy and by searching numerous sources, we may have failed to identify potentially relevant trials or experiments. For example, although we applied no language limits in our searches, the search for this review was based on English language. Thus, we may have missed some trials in other non-English databases. Also, publication bias might have occurred due to non-identification of unpublished trials. It is unclear how this may have affected our conclusions.
We planned to carry out a meta-analysis and additional subgroup analyses to identify program components associated with more effective outcomes and factors that modified intervention effectiveness; however, we included only one study. Similarly, with one study it was impossible to carry out sensitivity analyses to examine the impact of study design or quality.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the first systematic review assessing the difference between compared FM appliances with expansion only vs expansion and constriction.
A recent systematic review of early orthodontic treatment for Class III malocclusion was carried out (Woon and Thiruvenkatachari 2017). That review included 15 RCTS, only 3 were classified as having a low risk of bias and addressed effect of FM (with RME) versus no FM (Mandall et al. 2010, Mandall et al. 2012), FM versus tongue plate (Showkatbakhsh et al. 2013) and finally FM with Alt-RAMEC versus FM with RME (Liu et al. 2015). The latter study was the included RCT in this review. Among the conclusion of the authors was that moderate evidence to show that early treatment with a FM resulted in positive improvements in both skeletal and dental changes in the short term. However our assessment of the study (Liu et al. 2015) was that it has unclear risk of bias due to the reasons explained earlier.
Authors’ conclusions
Implications for practice
Despite a thorough search for evidence relating to the efficacy of treatment, no strong evidence was found to show that FM with alternate rapid palatal expansion and constriction is different from FM with rapid palatal expansion in correction of Class III malocclusion in the short term.
Implications for research
Given the absence of high quality evidence on the effectiveness of FM with Alt-RAMEC versus FM with RME, researchers must carefully consider the need for future RCTs. FM with Alt-RAMEC constriction typically takes around 7 weeks of alternate expansion and constriction, the burden of such treatment time and inconvenience for patients must be weighed against robust evidence of effectiveness of the procedure. Nonetheless, other systematic reviews have also failed to found evidence of difference between treatment with FM with and without RME on the effectiveness of treatment. Further research is required to identify difference in effectiveness between FM either Alt-RAMEC and RME and no RME.
Conclusions:
- There is low quality evidence from one RCT with a small sample size that ,in the short term, FM and Alt-RAMEC has a statistically significant forward maxillary movement, greater palatal plane counterclockwise rotation and less mandibular downward and backward rotation compared with RME and FM. These results might not be clinically significant though.
- There is a need for high quality RCT to identify any difference between FM with RME or with Alt-RAMEC, especially on the long term.
Contributions of authors
The review was coordinated by Aman Ulhaq (AU). Abdullah Alkalaly (AK) undertook the database search and the hand searching. AA, UA screened the search results and retrieved papers, appraised and agreed on the quality of the papers in consultation with BA (Bodore Albaker). AA, UA and BA extracted data from the papers. AA and AU analysed and interpreted the data. AU rechecked the data extraction and approved the final version of the study. AA, AU and BA wrote the review.
Declaration of Interest
Abdullah Alkalay: no interests to declare.
Bodore Albaker: no interests to declare.
Aman Ulhaq: no interests to declare.
Sources of funding
None
Differences between protocol and review
Average protraction time and total average treatment time were added as secondary outcomes.
References
Baccetti, T., J. S. McGill, L. Franchi, J. A. McNamara Jr and I. Tollaro (1998). “Skeletal effects of early treatment of Class III malocclusion with maxillary expansion and face-mask therapy.” American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 113(3): 333-343.
Cordasco, G., G. Matarese, L. Rustico, S. Fastuca, A. Caprioglio, S. J. Lindauer and R. Nucera (2014). “Efficacy of orthopedic treatment with protraction facemask on skeletal Class III malocclusion: A systematic review and meta-analysis.” Orthodontics and Craniofacial Research 17(3): 133-143.
da Luz Vieira, G. D. D. S. M. S., L. M. D. D. S. M. S. P. D. de Menezes, E. M. D. D. S. M. S. P. D. de Lima and S. D. D. S. M. S. Rizzatto (2009). “Dentoskeletal Effects of Maxillary Protraction in Cleft Patients With Repetitive Weekly Protocol of Alternate Rapid Maxillary Expansions and Constrictions.” Cleft Palate-Craniofacial Journal 46(4): 391-398.
Deeks, J. J., J. Higgins and D. G. Altman, (editors) (2017). on behalf of the Cochrane Statistical Methods Group.Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane handbook for systematic reviews of interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Egger, M., G. D. Smith, M. Schneider and C. Minder (1997). “Bias in meta-analysis detected by a simple, graphical test.” British Medical Journal 315(7109): 629-634.
GRADEpro GDT (2015). GRADEpro Guideline Development Tool [Software]. McMaster University, (developed by Evidence Prime, Inc.). Available from gradepro.org.
Higgins, J., D. Altman and J. e. Sterne (2017). Chapter 8: Assessing risk of bias in included studies In Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors) Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane , 2017. Available from www.training.cochrane.org/handbook.
Isci, D., T. Turk and S. Elekdag-Turk (2010). “Activation–deactivation rapid palatal expansion and reverse headgear in Class III cases.” The European Journal of Orthodontics 32(6): 706-715.
Kiliçoglu, H. and Y. Kirliç (1998). “Profile changes in patients with class III malocclusions after Delaire mask therapy.” American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 113(4): 453-462.
Kim, J. H., M. A. Viana, T. M. Graber, F. F. Omerza and E. A. BeGole (1999). “The effectiveness of protraction face mask therapy: a meta-analysis.” American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 115(6): 675-685.
Liberati, A., D. G. Altman, J. Tetzlaff, C. Mulrow, P. C. Gøtzsche, J. P. Ioannidis, M. Clarke, P. J. Devereaux, J. Kleijnen and D. Moher (2009). “The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.” PLoS medicine 6(7): e1000100.
Liou, E. (2005). “Toothborne orthopedic maxillary protraction in Class III patients.” Journal of clinical orthodontics: JCO 39(2): 68-75.
Liou, E. J.-W. and W.-C. Tsai (2005). “A new protocol for maxillary protraction in cleft patients: repetitive weekly protocol of alternate rapid maxillary expansions and constrictions.” The Cleft palate-craniofacial journal 42(2): 121-127.
Liou, E. J. (2005). “Effective maxillary orthopedic protraction for growing Class III patients: a clinical application simulates distraction osteogenesis.” Progress in Orthodontics 6(2): 154-171.
Liu, W., Y. Zhou, X. Wang, D. Liu and S. Zhou (2015). “Effect of maxillary protraction with alternating rapid palatal expansion and constriction vs expansion alone in maxillary retrusive patients: a single-center, randomized controlled trial.” American Journal of Orthodontics and Dentofacial Orthopedics 148(4): 641-651.
Mandall, N., R. Cousley, A. DiBiase, F. Dyer, S. Littlewood, R. Mattick, S. J. Nute, B. Doherty, N. Stivaros and R. McDowall (2016). “Early class III protraction facemask treatment reduces the need for orthognathic surgery: a multi-centre, two-arm parallel randomized, controlled trial.” Journal of Orthodontics 43(3): 164-175.
Mandall, N., A. DiBiase, S. Littlewood, S. Nute, R. Cousley, F. Dyer, R. Mattick, B. Doherty, N. Stivaros, R. McDowall, I. Shargill and H. Worthington (2010). “Is early class III protraction facemask treatment effective? A multicentre, randomized, controlled trial: 15-month follow-up.” Journal of Orthodontics 37(3): 149-161.
Mandall, N. A., R. Cousley, A. DiBiase, F. Dyer, S. Littlewood, R. Mattick, S. Nute, B. Doherty, N. Stivaros, R. McDowall, I. Shargill, A. Ahmad, T. Walsh and H. Worthington (2012). “Is early class III protraction facemask treatment effective?Amulticentre, randomized, controlled trial: 3-Year follow-up.” Journal of Orthodontics 39(3): 176-185.
Masucci, C., L. Franchi, V. Giuntini and E. Defraia (2014). “Short-term effects of a modified Alt-RAMEC protocol for early treatment of Class III malocclusion: A controlled study.” Orthodontics and Craniofacial Research 17(4): 259-269.
McNamara Jr, J. A. (1987). “An orthopedic approach to the treatment of Class III malocclusion in young patients.” Journal of clinical orthodontics : JCO 21(9): 598-608.
REVMAN (2014). Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rücker, G., G. Schwarzer and J. Carpenter (2008). “Arcsine test for publication bias in meta‐analyses with binary outcomes.” Statistics in medicine 27(5): 746-763.
Ryan, R. and S. Hill (2016). How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group, available at http://cccrg.cochrane.org/author-resources. Version 3.0 December 2016.
Schünemann, H. J., A. D. Oxman, G. E. Vist, J. P. Higgins, J. J. Deeks, P. Glasziou and G. H. Guyatt (2017). “on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.”.
Showkatbakhsh, R., L. Toumarian, A. Jamilian, A. Sheibaninia, M. Mirkarimi and T. Taban (2013). “The effects of face mask and tongue plate on maxillary deficiency in growing patients: a randomized clinical trial.” Journal of Orthodontics 40(2): 130-136.
Turley, P. K. (2007). “Treatment of the Class III Malocclusion with Maxillary Expansion and Protraction.” Seminars in Orthodontics 13(3): 143-157.
Vaughn, G. A., B. Mason, H.-B. Moon and P. K. Turley (2005). “The effects of maxillary protraction therapy with or without rapid palatal expansion: a prospective, randomized clinical trial.” American Journal of Orthodontics and Dentofacial Orthopedics 128(3): 299-309.
Wang, Y.-C., P. M. Chang and E. J.-W. Liou (2009). “Opening of circumaxillary sutures by alternate rapid maxillary expansions and constrictions.” The Angle orthodontist 79(2): 230-234.
Watkinson, S., E. Harrison Jayne, S. Furness and V. Worthington Helen (2013) Orthodontic treatment for prominent lower front teeth (Class III malocclusion) in children. Cochrane Database of Systematic Reviews DOI: 10.1002/14651858.CD003451.pub2
Woon, S. C. and B. Thiruvenkatachari (2017). “Early orthodontic treatment for Class III malocclusion: A systematic review and meta-analysis.” American Journal of Orthodontics and Dentofacial Orthopedics 151(1): 28-52.
Yavuz, İ., K. Halicioglu, İ. Ceylan, I. M. Dagsuyu and A. Erdem (2012). “The effects of face mask therapy with and without rapid maxillary expansion in adolescent patients.” Australian orthodontic journal 28(1): 63.
Glossary
Attrition:Often participants are lost during a trial and cannot be included in the analysis. This is termed attrition or is sometimes known as mortality.
Bias:A term denoting that a known or unknown variable (rather than the intervention) is, or may be, responsible for an observed effect.
Concealedallocation:This is where the researchers, participants and teachers are prevented from knowing in advance the allocation of an individual, i.e. the allocation has been concealed from them.
Confidenceintervals:These indicate the level of uncertainty surrounding an effect size. The point estimate of effect of any intervention will always be imprecise. The level of the imprecision is dependent upon the sample size and event rate in the treatment groups. The use of confidence intervals (usually 95%, but sometimes 99% or 90%) reflects this imprecision in the study results.
Controlledtrial(CT):This usually means a study with a control group that has been formed by means other than randomization. Consequently, the validity of the study using this design is potentially threatened by selection bias.
Co-variatesorconfounders:These are variables that are associated with outcome. Randomization is the only method that ensures that both known and unknown co-variates are equally distributed among treatment groups.
Effectsize:When an outcome variable is measured on a continuous scale (e.g. changes in a test score) the improvement or decrement is described in standard deviation units, which is termed the effect size.
Funnelplot:A method of assessing whether there is any publication bias. The effect size of each study is plotted against its sample size. Small studies will have large random variations in their effect sizes, which will be scattered along the x-axis close to the bottom of the y-axis. Larger studies will be higher up on the y-axis and less scattered along the x-axis. A review with no publication bias will show a plot in the shape of an inverted funnel.
Heterogeneity:When studies have different characteristics, e.g. different populations or different outcome measures.
ITTanalysis:Intentiontoteachanalysis:This is where all participants are analysed in their original randomized groups; it is the most robust analytical method. Once participants have been allocated to their respective groups it is important that they remain in those groups for analysis, to avoid bias. A common, but incorrect, method is to exclude some participants after randomization for a variety of reasons. One approach is to do what is termed ‘an on- treatment analysis’ – this is where only those participants who demonstrate treatment fidelity are included in the analysis. Unfortunately, this can lead to bias, as those participants who complete treatment are likely to be different from those who do not. Intervention-received analysis can therefore produce a biased result.
Meta-analysis: A meta-analysis is a method of combining the results of two or more RCTs statistically.
Meta-analysis:fixedeffectsmodel:The fixed effects model of meta-analysis assumes that the variability is exclusively because of random sampling variation around a fixed effect.
Meta-analysis:randomeffectsmodel:The random effects model of meta-analysis assumes a different underlying effect for each study, and takes this into consideration.
Publicationbias:Not all RCTs are published. There is a well-established tendency for trials that produce negative or null effects to be less likely to be published than positive trials. Unless a systematic review includes these negative trials it can give a misleading optimistic assessment of the intervention. Existence of publication bias can be detected by using funnel plots.
PRISMA: preferred reporting items for systematic reviews and meta-nalyses
RandomizedControlled Trial (RCT):This is where two or more groups have been formed through random allocation (or a similar method). This is the only method that ensures that selection bias is eliminated at baseline.
Samplesizecalculations:Trials in educational research commonly exhibit a Type II error. This is where the sample size is insufficient to show, as statistically significant, a difference that is educationally important. Reviews of educational interventions have shown that most interventions will, at best, only lead to an improvement in the region of half a standard deviation and quite often somewhat less. Statistical theory shows that to reliably detect (with 80% power) half a standard deviation difference as statistically significant (p = 0.05) for a normally distributed variable requires a minimum sample size of 126 participants between the two arms. Studies that are smaller than this risk erroneously concluding that there was not a significant difference when actually there was. Therefore, a good quality study ought to describe the reasoning behind the choice of sample size.
Standarddeviation:is a measure of spread or dispersion of continuous data. A high standard deviation implies that the values are widely scattered relative to the mean value, whilst a small value implies the converse.
Systematicreview:A review where explicit methods have been used to identify, select and include studies fitting pre-specified criteria.
* Definitions reproduced from Torgerson (2003, pp.vii-x)
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Dentistry"
Dentistry is a branch of medicine that involves treating and preventing issues relating to your teeth, gums, and jaw. Dentistry not only ensures that you have a healthy mouth, but it can also have a positive impact on your general health and well-being.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




