Molecular Sieve Materials: Application in Adsorptive Gas Separation Processes
Info: 4901 words (20 pages) Dissertation
Published: 29th Nov 2021
Tagged: Chemistry
Abstract
The objective of this research was to develop new molecular sieve materials and to study their applications in membrane as well as adsorptive gas separation processes.
Membrane based processes have the potential to surpass the limitations of conventional gas separation techniques such as energy intensiveness, environmental concerns and possibly affordability. Natural zeolite membranes have recently been shown to demonstrate potential in separation of H2 from H2/CO2 mixtures or H2/light hydrocarbon mixtures and can be utilized to develop high performance molecular sieve membranes with advanced separation characteristics. In the previous work of this research team, disk membranes produced from dense natural clinoptilolite zeolite rocks showed high performance in gas separation. In this work, membranes from natural clinoptilolite powders are designed, studied and scaled up in disk and tubular configurations for gas separation applications. The membranes’ permeation, separation performance and separation mechanisms were evaluated using different characterization methods and tests at several operating conditions.
The results showed that natural zeolite membranes such as compact disk or coated stainless steel tubular ones, have great potential for large-scale gas separation at high temperature and pressures. To evaluate the potential of membranes in industrial applications, single versus multi-component gas permeance was compared and discussed.
In another study, a new adsorbent for the adsorptive separation of ethylene from ethane as one of the most energy intensive separations was created by incrementally changing the pore size of clinoptilolite. The structure of a naturally occurring clinoptilolite was modified through ammonium exchange, calcination, and post-calcinations steam treatment. The results demonstrated the potential to use steamed clinoptilolite and to increase the efficiency of the adsorptive separation of ethane/ethylene.
Results of this work suggest that natural zeolites can be employed as high performance membranes and be modified as unique adsorbents for enhanced gas separation purposes. With further research, natural zeolites can be developed into economically viable membranes and adsorbents for several industrial applications.
Preface
Most of the research conducted for this thesis is in collaboration with NOVA Chemicals Corporation (Center for Applied Research) in Calgary and it was led by the principal investigator, Dr. Steven M. Kuznicki, at the University of Alberta,
The technical apparatus referred to in chapter 5-7 were designed by this thesis author (Afrooz Farjoo) with the support of Weizhu An at the University of Alberta. The data analysis and conclusion are Afooz Farjoo’s work, as well as the literature review in chapters 1-2 and 4.
Chapter 3 of this thesis has been published as A. Farjoo, J. Sawada, and S. Kuznicki, “Manipulation of the pore size of clinoptilolite for separation of ethane from ethylene” Journal of Chemical Engineering Science, 2015, 138, 685-688. In this paper, Afrooz Farjoo was responsible for the data collection and analysis as well as the manuscript preparation. James Sawada contributed to manuscript correction and review. Dr. Steven. M. Kuznicki was the supervisory author and the principal investigator that was also involved with concept formation and manuscript review.
Chapter 5 of this thesis has been accepted for publication in Canadian Journal of Chemical Engineering as A. Farjoo, S. Adamaref, A. Avila and S. Kuznicki, “H2 separation using pressed clinoptilolite and mixed copper-membranes”. In this paper, Afrooz Farjoo was responsible for the data collection and data analysis as well as the manuscript preparation. Adolfo Avila contributed to manuscript preparation and correction. Solmaz Adamaref contributed to part of the data collection and Dr. Steven. M. Kuznicki was the supervisory author, the principal investigator, involved with concept development and the manuscript review.
Chapter 6 of this thesis has been accepted for publication in Canadian Journal of Chemical Engineering as: Afrooz Farjoo, Steven Kuznicki, “H2 separation using tubular stainless steel supported natural clinoptilolite membranes”, In this paper, Afrooz Farjoo was responsible for the data collection and analysis as well as the manuscript preparation. Dr. Steven. Kuznicki was the supervisory author, the principal investigator and was involved with concept development, and the manuscript review.
Chapter one: Introduction to Zeolites Molecular Sieves
1.1 Zeolites Molecular Sieves
Zeolite molecular sieves are microporous crystalline metal oxides that offer a unique arrangement for separating molecules according to their shape and size [1]. Zeolites are synthetic or natural crystalline aluminosilicates with three-dimensional framework structures and uniform pore sizes. Molecular sieve zeolites have been commercially utilized in several processes as catalysts, adsorbents, ion-exchange and purification agents. Classical zeolites and mixed coordination materials also identified as inorganic crystalline molecular sieves have broad industrial applications.
Classical zeolites’ structures are four coordinated aluminum and silicon chains in the form of AlO4 and SiO4 tetrahedral units. Silica units are not charged and one negative charge is associated with aluminum units to sustain electron neutrality. The most recognized examples of commercial zeolites are: zeolite A, zeolite X and zeolite Y [2] .
Originally the majority of zeolites were natural minerals and were formed when volcanic rocks and residues reacted with alkaline ground water or in particular post-depositional conditions for the duration of thousands to millions of years in low marine basins [3],[4]. Examples of natural zeolites are clinoptilolite, chabazite, mordenite, erionite, ferrierite and phillipsite, among them clinoptilolite and chabazite and are largely in use for industrial gas separation [5]. High thermal stability, abundance, and low price of the raw material, outweigh some features of natural zeolites such as non-uniformity and low purity. Adsorptive and transport properties in zeolites can be modified by ion exchange, structural changes or thermal treatment to enhance their separation potentials [6].
The micro-porous frameworks in mixed coordination molecular sieves have interconnected octahedra and tetrahedra. They also have rings of 4-coordinated tetrahedral silicon atoms connected to parallel chains of 6-coordinated octahedral titanium atoms. While silica units are not charged, titanium chains are associated with the “-2” structural charge. One of the distinguished examples of mixed coordination molecular sieves is the Engelhard Titanium Silicate (ETS-4, ETS-10) [7].
The pore space in zeolites is in the range of 0.3 nm to 1.0 nm which is comparable with the kinetic diameter of gas molecules. This characteristic makes zeolite materials potential candidates in gas separations based on differences in molecular kinetic diameter which is identified as the restrictive cross sectional molecular dimension. Table 1-1 summarizes kinetic diameter for some of the gases used in this study.
Table 1-1. Kinetic Diameter of some gases molecules [2],[8],[9]
| Gas | Kinetic Diameter (nm) |
| Helium (He)
Hydrogen (H2) Nitrogen (N2) Oxygen (O2) Methane (CH4) Ethane (C2H6) Ethylene (C2H4) n-butane (n-C4H10) Carbon dioxide (CO2) |
0.26
0.28 0.36 0.34 0.38 0.41 0.44 0.43 0.33 |
The steric effect originates from the molecular sieving characteristic of zeolites according to the uniform pore size in crystalline structure. Molecular exclusion is based on the diversity in molecular pore size. Only suitably shaped molecules can diffuse into the adsorbent while other molecules are entirely excluded. Titanosilicate ETS-4 with a typical pore size in the range of 3-5Å that can be tailored to exclude methane with a kinetic diameter of 3.8 Å from nitrogen with a kinetic diameter of 3.6 Å [10] .
1.2 Natural Zeolites
Zeolites have been known for over two centuries and initially their properties were not considered as unique ones. Commercial applications of natural zeolites are relatively recent and have received increasing research interest over the past decades. Current categorization and application of natural zeolite crystals have become an interdisciplinary study that involves physics, chemistry, petrology, geology and engineering [4]. The common natural zeolite frameworks’ formula is as follows [4]:
(Li+, Na+, K+)a (Mg++, Ca++, Sr++, Ba++)d [Al(a+2d)Sin-(a+2d)O2n].m H2O
The component of the formula in square brackets represents the tetrahedral framework of the zeolite with a negative structural charge [4]. Transferable cations are critical to balance the structure with negative charge. Cations in the above formula, split by commas in round brackets, show that they are transferable with one another [4]. A monovalent cation may exchange with another monovalent cation and the same approach applies for divalent cations. A single monovalent cation cannot substitute a single divalent one. Keeping charge neutrality requires two monovalent cations to substitute a single divalent cation. The compositions of naturally occurring zeolites vary extensively with the primary constraint of Si ≥ Al, as it is possible to replace only each second silica with aluminum in the tetrahedral framework [2].
1.2.1 Clinoptilolite and Heulandite
The heulandite framework (HEU) is considered as one the most common naturally occurring zeolitic frameworks [10], [12]. The HEU framework has parallel 8- and 10-member rings with a cross channel that contains 8-member ring [13],[14]. The parallel 8- and 10- member rings have pore diameters of 4.1-4.7 Å and 4.4-7.2 Å respectively [13]. The 8 member-ring cross channel has a pore diameter of 4.0-5.5 Å [13]. Synthesizing HEU materials in the laboratory is technically challenging, therefore, less pure and as a result less expensive natural materials are used more commonly [14], [15]. Clinoptilolite is a natural zeolites from the heulandite category [16]. Clinoptilolite and heulandites, both share the HEU structural framework. In 1822, HEU was called after H. Heuland, the English mineralogist [4]. Clinoptilolite was known more than a century after heulandites in 1932 [17]. It was given the name “clinoptilolite” thanks to its distinguishing inclined edges and similarity to mordenite [17]. Currently, both heulandite and clinoptilite have industrial applications because of their low price and abundance while they still propose the unique features of zeolitic materials [4,11,17]. The formulas for clinoptilolite and heulandite are as follows [13]:
Heulandite: (Na+, K+)1 (Ca++)4 [Al9Si27O72] 24H2O
Clinoptilolite: (Na+, K+)6 [Al6Si30o72].20 H2O
One approach to distinguish different types of zeolites such as clinoptilolite and heulandites is comparison of the cationic content [18]. Yet this measure is not necessarily valid when clinoptilolite and heulandite change into one another through different processes such as ion exchange. The Structural Commission of the International Zeolite Association (IZA) assigns the HEU code to both of the clinoptilolite and heulandite framework structures, however heulandite acquired the HEU code earlier than clinoptilolite as it was found first [8].
Both Heulandite and clinoptilolite share the same HEU framework. IZA defines zeolite structures as clinoptilolite when the Si/Al ratio is larger than 4, and as heulandites when this ratio is less than 4 [8]. Clinoptilolite framework structure has a 2-dimensional system of intersecting channels that are 8- or 10- member rings [A (c-axis, 4.4 × 7.2Å), B (c-axis, 4.7 × 4.1Å) and C (a-axis, 5.5×4.0Å)] [2]. Figure 1-1 shows the HEU unit cell structure.
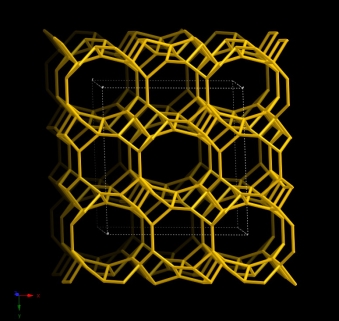
Figure 1-1. Unit cell structure of the HEU framework (Viewed alongside [001])
[Database of Zeolite Structures: america.iza-structure.org/IZA-SC]
1.3 Synthetic Zeolites
Zeolite A is believed to be the first synthetic zeolite that was formed in corrosive conditions through gradual crystallisation of silica-alumina gel. Zeolite A was first announced as a synthetic zeolite in 1948 by Milton [19].
Since 1948, more than 100 synthetic zeolites have been developed for many of which there is no natural comparable zeolite. Crystal sizes of synthetic zeolite are most likely smaller than their natural comparables, however there are more pure and uniform, making them unique candidates as catalysts or as adsorbents [1, 2, 19]. Synthesis processes in zeolites are described in detail by Cundy [1].
Natural or synthetic zeolites are employed commercially in catalytic reactions, adsorption, ion exchange and membrane separation applications [19].
This work focuses on developing novel molecular sieve materials and applying them in adsorptive gas separation in particular for energy intensive separations such as ethane and ethylene as well as developing inorganic ceramic membranes for gas separation processes. Chapter two reviews the fundamentals of adsorption and adsorptive gas separation processes. Chapter three describes the kinetic separation of ethylene and ethane and introduces a novel adsorbent for exclusive separation of ethane and ethylene.
After reviewing membranes and fundamentals of gas separation in membranes in chapter four, chapters five to seven focus on coated composite zeolite membranes in different configurations such as disk or tubular in addition to single against multi-component gas separation mechanisms in membranes. Finally, the dissertation is finished with chapter eight, which highlights the key outcomes of this research program and recommends the potential future work.
1.4 References
[1] C. S. Cundy and P. a. Cox, “The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism,” Microporous Mesoporous Mater., vol. 82, no. 1–2, pp. 1–78, 2005.
[2] D.W. Breck, “Zeolite Molecular Sieves: Structure, Chemistry, and Use,” no. John Wiley & Sons, Ltd, 1974.
[3] M. W. Ackley, S. U. Rege, and H. Saxena, “Application of natural zeolites in the purification and separation of gases,” Microporous Mesoporous Mater., vol. 61, pp. 25–42, 2003.
[4] G. Gottardi and E. Gallim, “Natural Zeolites“, Germany: Springer-Verlag Berlin Heidelberg, 1985.
[5] W. Ackley, Mark, S. U. Rege, and H. Saxena, “Application of natural zeolites in the purification and separation of gases”, Microporous Mesoporous Mater., vol. 61, pp. 25–42, Jul. 2003.
[6] A. W. Thornton, T. Hilder, A. J. Hill, and J. M. Hill, “Predicting gas diffusion regime within pores of different size, shape and composition”, J. Memb. Sci., vol. 336, pp. 101–108, Jul. 2009.
[7] Steven Kuznicki and Kathleen A. Thrush, “Large pored molecular sieves with charged octahedral titanium and charged tetrahedral aluminum”, US005244650A, 1993.
[8] L. B. Mccusker and D. H. Olson, “Atlas of Zeolite Framework Types”. Elsevier, 2007.
[9] S. M. Kuznicki, V. Bell, S. Nair, H. W. Hillhouse, R. M. Jacubinas, C. M. Braunbarth, B. H. Toby, and M. Tsapatsis, “A titanosilicate molecular sieve with adjustable pores for size-selective adsorption of molecules”, Nature, vol. 412, no. 6848, pp. 720–4, Aug. 2001.
[10] F. A. Mumpton, “Mineralogy and Geology of Natural Zeolites“, Washington, D.C.: Mineralogical Society of America,. 1977.
[11] W. An, P. Swenson, L. Wu, T. Waller, A. Ku, and S. M. Kuznicki, “Selective separation of hydrogen from C1/C2 hydrocarbons and CO2 through dense natural zeolite membranes”, J. Memb. Sci., vol. 369, no. 1–2, pp. 414–419, Mar. 2011.
[12] D. L. Bish and M. D.W., “Natural Zeolites: Occurrence, Properties, Application”, Rev. Miner. Geochemistry, vol. 45, 2001.
[13] D. Zhao, K. Cleare, C. Oliver, C. Ingram, D. Cook, R. Szostak, and L. Kevan, “Characteristics of the synthetic heulandite-clinoptilolite family of zeolites”, Microporous Mesoporous Mater., vol. 21, no. 4–6, pp. 371–379, 1998.
[14] S. Satokawa and K. Itabashi, “Crystallization of single phase (K , Na) -clinoptilolite,” Microporous Mater., vol. 8, pp. 49–55, 1997.
[15] A. Ansón, S. M. Kuznicki, T. Kuznicki, T. Haastrup, Y. Wang, C. C. H. Lin, J. A. Sawada, E. M. Eyring, and D. Hunter, “Adsorption of argon, oxygen, and nitrogen on silver exchanged ETS-10 molecular sieve”, Microporous Mesoporous Mater., vol. 109, no. 1–3, pp. 577–580, 2008.
[16] W. T. Schaller and U. S. Geological, “The Mordenite-Ptilolite Group; Clinoptilolite, a New Species”, Mineral. Soc. Am., vol. 8, pp. 128–134, 1923.
[17] A. Julbe, “Zeolite membranes-synthesis, charactersitic and application” in “Intorduction to zeolite science and practices”, Elsevier B.V., 2007.
[18] A. Dyer, “An Introduction to Zeolite Molecular Sieves”, John Wiley, Chichestar, 1988.
[19] R. Szostak, “Handbook of Molecular Sieves: Structures”, New York: Van Nostrand Reinhold, 1992.
Chapter Two: Fundamentals of adsorption and adsorptive gas separation processes
2.1 The Theory of Adsorption
Adsorption is an impulsive thermodynamic process taking place when liquid or gas molecules build up on the surface of a solid adsorbent and form a film of atoms or molecules identified as adsorbate. In gas adsorption process on porous materials such as zeolite molecular sieves, the gas phase is essentially concentrated in the interior channels of the porous framework where surface adsorption may also happen.
Adsorption that is associated with the diffusion of a substance into the solid materials is a result of surface energy and is different from absorption. In adsorption, a gas molecule close to a solid surface experiences low potential energy as a consequence of interaction with atoms or molecules in the solid phase. As a result, the gas molecules have a tendency to concentrate in solid region where the molecular density in the area of the surface is significantly larger than the gas phase [1], [2].
The effectiveness of the surface forces depends on the nature of the adsorbate and the adsorbent. If the forces are comparatively weak (only Van der Waals interactions) complemented with polar or quadrupolar groups by electrostatic forces (e.g. dipole or quadrupole interactions, etc), it is recognized as physical adsorption [1]. If the interaction forces are strong and involve a major extent of electron transfer, it is recognized as chemical adsorption which is restricted to a monolayer, while in physical adsorption, multiple molecular layers can also be formed [2].
Gas adsorption is commonly used in chemical processing applications for gas separation and purification. Examples of solid adsorbents in adsorptive gas separation are activated carbons, zeolite molecular sieves, activated alumina, silica gels, and synthetic resins.
2.2 Fundamental Mechanisms of Gas Separation
Adsorptive separation of gas mixtures on porous solids such as zeolites is mainly by one of the below major separation mechanisms or a combination of some of them [4], [5]:
- Equilibrium Separation
- Steric Separation
- Kinetic Separation
2.2.1 Equilibrium Separation
Equilibrium separation is the most common mechanism of adsorptive gas separation. It is based on the differences in the interaction between the competitive adsorbates and the adsorbent controlled by thermodynamics and electrostatics laws.
Equilibrium selectivity and capacity are the key features associated with equilibrium separation. While the equilibrium selectivity is the separation factor at equilibrium conditions, the capacity of the adsorbent depends on two corresponding factors of surface area and porosity.
An example of equilibrium separation is oxygen production from air by means of Li-LSX zeolite [6] . Li-LSX with considerably higher affinity for nitrogen than oxygen separates a pure flow of oxygen when air passes through the zeolite bed as adsorbent.
2.2.2 Kinetic Separation
Kinetic separation is based on the difference in the adsorption rate than equilibrium affinities when one adsorbate group adsorbs quicker than the competitors. The rate of physical adsorption is typically controlled by diffusion restrictions rather than the real rate of equilibration on a surface. Therefore, many equilibrium gas separation processes are not exclusively equilibrium-based but include a kinetic component associated with mass transfer which is known as “quasi-equilibrium” [7], [8].
Carbon molecular sieves (CMS) correspond to a class of adsorbents that separate molecular groups through the kinetic phenomenon. Kinetic separation is achievable with CMS since it has a range of pore sizes. Such a distribution of pores allows different gases to diffuse at different rates while completely avoiding exclusion of other gases in the mixture. Another example of kinetic separation using carbon molecular sieves is the production of nitrogen from air. The diffusion of oxygen is 30 times faster than nitrogen diffusion in CMS even if the capacity of CMS is comparatively no more than just a fraction of most zeolites. It might appear more reasonable to use CMS for the production of nitrogen from air [9],[10].
2.2.3 Steric Separation
The steric effect is based on the theory that only suitably oriented and small molecules can diffuse into the pores of the adsorbent, whereas other molecules, either because of size or geometry are entirely excluded. Steric separation is originated from the molecular sieving property of zeolites. This is a feature of zeolitic adsorbents since these materials are crystalline and the size of the micropores are determined by the crystal structure. Control of pore size in zeolites can be achieved through cation-exchange method [11], framework anion replacement [12] or structural contraction through de-alumination and steaming processes [13].
2.3 Adsorptive Gas Separation Processes
Adsorptive gas separation processes are classified by their method of regeneration process. Pressure swing adsorption (PSA) and temperature swing adsorption (TSA) are the more commonly employed processes for gas separation. However, other processes employ purge swing cycles or reactive sorption. Most adsorptive processes use fixed beds while some utilize moving fluidized beds or rotary wheels [14].
2.3.1 Adsorbent Design
Adsorbent in adsorptive gas separation processes offers a particular surface for the selective adsorption of specific gas molecules [10]. A high selectivity is the principal requirement, while a high capacity is also essential to conclude the size of the adsorbent bed and the regeneration process. Since the overall performance of an adsorptive gas separation process depends on both equilibrium and kinetic factors, adsorbent design ought to consider equilibrium properties such as selectivity and capacity as well as the diffusion rates.
2.3.2 Pressure Swing Adsorption
As thermodynamics favor adsorption at higher operating pressures, a pressure swing adsorption cycle is one in which desorption takes place at a pressure much lower than adsorption cycle. Its primary application is for mass separations where contaminants are present at high concentrations. Systems with weakly adsorbed groups are particularly suitable for PSA cycles. Examples of PSA separation are air separation and upgrading of fuel gases.
Pressure swing adsorption (shown in Figure 2.1) can be further categorized into three categories: classical PSA, vacuum swing adsorption (VSA), and vacuum-pressure swing adsorption (VPSA). The main distinction between these three processes is the operating pressure choice. A classical PSA cycle swings between a high super-atmospheric (e.g. above 1 atm) and a lower super-atmospheric pressure. A VSA cycle swings from a super-atmospheric pressure to a sub-atmospheric (e.g. below 1 atm) pressure. VPSA cycles swing quickly and immediately above and below atmospheric pressure, as a result, it is the most efficient of all the above mentioned PSA categories.
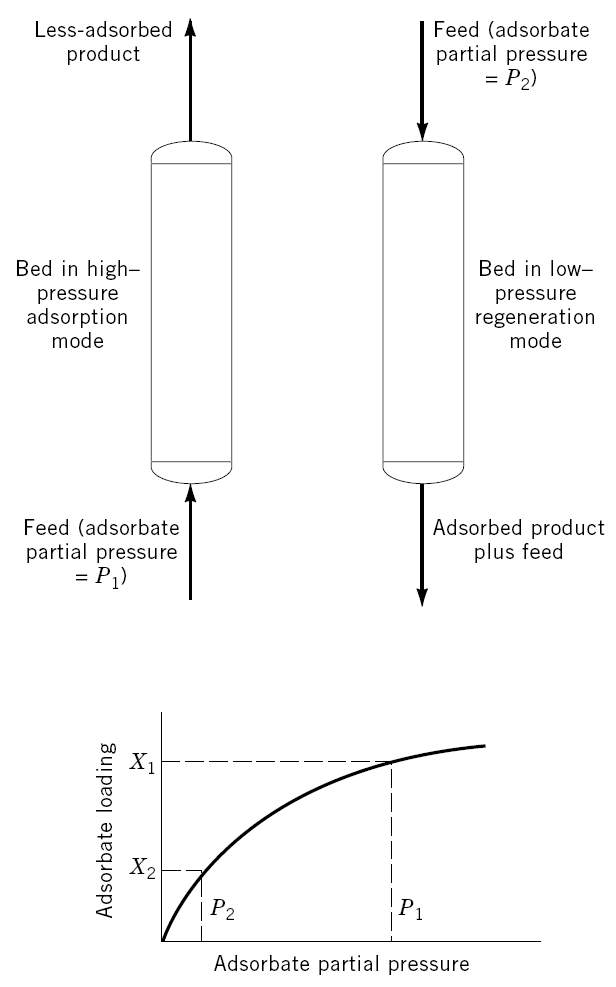
Figure 2-1. Schematic picture of a pressure swing adsorption cycle [2].
2.3.3 Temperature Swing Adsorption
As thermodynamics favor adsorption at lower operating temperatures, a temperature swing adsorption cycle is a process in which desorption takes place at a temperature much higher than adsorption. Its main application is for separation at low concentrations (trace-separation) where low concentration of contaminants exists. Systems with strongly-adsorbed groups are particularly suitable for TSA cycles. The applications of TSA (shown in Figure 2-2) separation include sweetening, desiccation, pollution control and carbon dioxide removal [14].
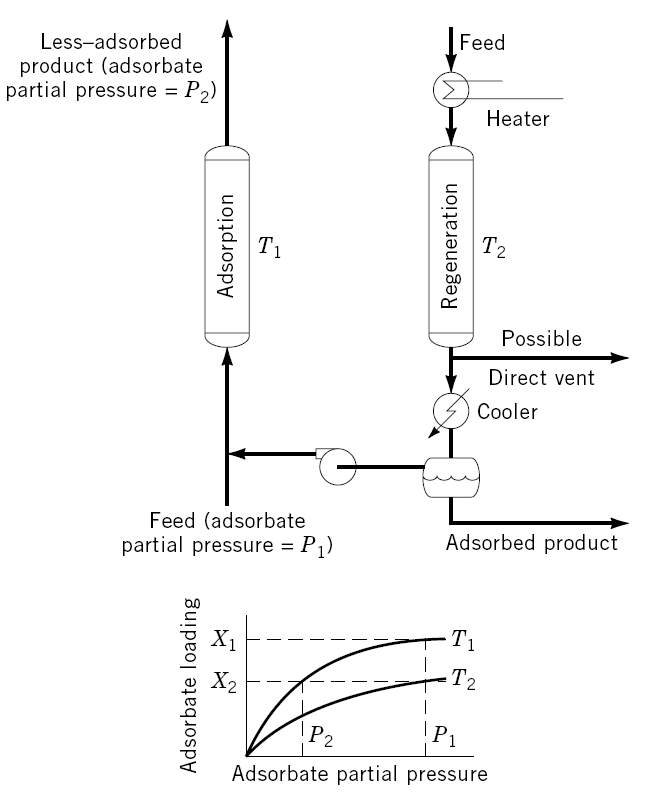
Figure 2-2. Schematic picture of a temperature swing adsorption cycle [2].
2.4 Henry’s Law
The adsorbed layer at the surface of adsorbents thermodynamically might be considered as an individual phase. Equilibrium with the nearby gas or liquid is described by the laws of thermodynamics. Physical adsorption in the gas phase is an exothermic in which equilibrium favors adsorption at low temperatures and desorption at high temperatures. At adequately low pressures, the equilibrium typically approaches a liner trend known as the Henry’s law [15]:
v = KP (1)
Where the proportionality constant (K) is the Henry’s law constant and it decreases with rising temperature. The temperature reliance of the Henry’s law constant can be described in terms of the Van’t Hoff formula[15]:
K= A. exp (-∆HRT) (2)
Where A is the pre-exponential factor, ΔH is the heat of adsorption, R is the gas constant, and T is the absolute temperature.
2.5 Selectivity
The separation factor or selectivity in adsorptive gas separation is described as adsorbent’s preference to the completing the adsorbates. The limiting selectivity (α) or selectivity of gas A over gas B in the Henry’s law region, is identified as the ratio of the Henry’s law constants of gas A and gas B:
α(A/B) =
KAKB (3)
2.6 Heat of Adsorption
Physical adsorption processes are in nature exothermic, as the entropy change (ΔS) and the free energy change (ΔG) are both negative for the duration of the adsorption process. Therefore, thermodynamics would require the enthalpy change (ΔH), or the heat of adsorption to be negative or exothermic.
The isosteric heat of adsorption, δHiso, is determined from the slope of the adsorption isostere which is a constant adsorbate loading on the graph of ln P vs. 1/T by Clausius-Clapeyron equation [15]:
dlnPd(1T)= ∂HisoR (4)
Where, R is the gas constant, P is the adsorbate absolute pressure, and T is the absolute temperature.
2.7 Conclusion
Performance of an adsorptive gas separation process depends on material development as the separating agent and adsorption operating conditions that determines the mechanism. Development of new microporous materials is crucial to the chemical processing industry, energy intensive gas separations and the innovation of next-generation adsorptive gas separation applications.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Chemistry"
Chemistry is a science involving the study of the elements and matter at the atomic and molecular level including their composition, structure, properties, behaviour, and how they react or combine.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




