Examination of Laminar Diffusion Flames
Info: 10980 words (44 pages) Dissertation
Published: 10th Dec 2019
Tagged: Engineering
Contents
2.1 Combustion and Thermochemistry
2.1.1 Basis Property Relations
Extensive and Intensive Properties
2.1.2 First Law of Thermodynamics
2.1.4 Adiabatic Flame Temperature
Factors that Affect Reaction Rate
Design and Fabrication of a Laminar Diffusion Flame Test Rig
Fuels and Fuels Blending for Test
Chromatographic Analysis of Fuels
CHAPTER 1
INTRODUCTION
Background of Study
Even though considerable progress of the renewable energy development has been recently achieved, for the coming decades, hydrocarbons will continue to be the primary energy source of the modern society. In particular, it is expected that liquid hydrocarbon fuels will continue to dominate the transportation sector due to their high energy efficiency and their facility to be transported and stocked. Nevertheless, the necessity to drastically reduce pollutant emissions from ground and aero transportation engines becomes a relevant aspect in designing combustion systems such as helicopter turbines, aircraft or automotive engines. Since the combustion behavior of liquid hydrocarbons has a strong influence on engine performances, a better understanding of liquid fuel combustion is still a key point in developing high efficiency and clean-burning engines of next generation (Yi, 2017).
The highly complex nature of hydrocarbon diffusion flames poses challenges in unraveling the underpinning physical and chemical mechanisms of soot formation and oxidation. As a result, only a few principles are firmly established mostly for atmospheric gaseous fuel diffusion flames. For liquid fuels, we still rely on smoke point of the fuel or sooting index for practical applications due to a lack of full understanding of the effects of the various operating conditions on the soot formation process (Karatas, Intasopa, & Gülder, 2013).
In real life situations combustion occurs in air and is not ideal. The ideal equation assumes all of the reactant fuel reacts with air to produce carbon dioxide and water, with the nitrogen remaining unchanged. Real life combustion occurs at high temperatures, producing nitrogen oxide compounds and is almost always incomplete, leaving partially and unburned hydrocarbons in the exhaust gases, as well as soot. A measure of these particular emissions in conjunction with engine performance could help categorise biofuel efficiency and applicability (Martin, 2013).
In practical diffusion combustion systems, such as diesel and aircraft gas turbine engines, and in fires the combustion is turbulent. However, the high level of intermittency and relatively shorter residence times associated with turbulent diffusion flames limit the experimental accessibility of these flames and make it difficult to track combustion events like soot formation. Further the non-homogeneous nature of turbulent diffusion flames makes it challenging to isolate parameters that affect soot formation and oxidation (Yi, 2017).
Aim and Objective
The purpose of this project is to identify the effects that fuel type, fuel blending, fuel volume and wick type have on soot formation through the examination of laminar diffusion flames. An analysis such as this can be used as the basis for determining the efficiency and applicability of both petro and biodiesel fuels.
Scope of Study
- Design and fabrication of a laminar diffusion flame test rig.
- Fuels and fuels blending for test.
- Chromatographic analysis of fuels used.
- Test matrices
- Effects of blending
- Effects of fuel volume
- Effects of wick type
- Methods for calculating soot volume fraction
Project Layout
This project is organised as follows: Chapter 2 includes the scientific background and the literature review. The methodology and experimental setup are discussed in chapter 3. Chapter 4 presents the data analysis while the results of the experiment and further discussion are in chapter 5. Chapter 6 presents the conclusions and recommendations for future work.
CHAPTER 2
LITERATURE REVIEW
2.1 Combustion and Thermochemistry
Thermochemistry deals with the study of the energy and the heat related with chemical reactions and physical alterations. A reaction may undergo the release or absorption of energy, and a phase change may occur, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the system‘s energy exchange with its surroundings. Thermochemistry is useful in predicting reactant and product quantities throughout the course of a given reaction. In combination with entropy determinations, it is also used to predict whether a reaction is spontaneous or non-spontaneous, favorable or unfavorable.
Endothermic reactions absorb heat, while exothermic reactions release heat. Thermochemistry coalesces the concepts of thermodynamics with the concept of energy in the form of chemical bonds. The subject commonly includes calculations of such quantities as heat capacity, heat of combustion, heat of formation, enthalpy, entropy, free energy, and calories. (Wikipedia, https://en.wikipedia.org/wiki/Thermochemistry, 2016)
Combustion can be defined as a chemical reaction between two or more substances, usually involving oxygen and accompanied by the evolution of energy in the forms of heat, sound and light. Flames are the visual physical form of propagation. “The rate or speed at which the reactants combine is high in part because of the nature of the chemical reaction itself and in part because more energy is generated than can dissipate into the surrounding medium, with the result that the temperature of the reactants is raised to accelerate the reaction even more. A familiar example is a lighted match. When a match is struck, friction heats the potassium chlorate to a temperature at which the it reacts and generate more heat than can escape into the air, and they burn with a flame. If a wind blows away the heat or the chemicals are moist and friction does not raise the temperature sufficiently, the match goes out. Properly ignited, the heat from the flame raises the temperature of a nearby layer of the matchstick and of oxygen in the air adjacent to it, and the wood and oxygen react in a combustion reaction. When equilibrium between the total heat energies of the reactants and the total heat energies of the products (including the actual heat and light emitted) is reached, combustion stops” (Britannica.com, n.d.).
2.1.1 Basis Property Relations
Extensive and Intensive Properties
Extensive properties depend on amount of substance considered. Usually denoted by capital letters. Examples are: V for volume, U for total internal energy, H for total enthalpy.
“Intensive properties are expressed per unit amount of substance (mass or mole). Their numerical values are independent of the amount of substance present. Usually denoted with lower case letters. Examples are: specific volume v, specific enthalpy h, specific heat cp. Important exceptions to this lower case convention are temperature T and pressure P. Molar based properties will be denoted by an overbar” (Kirillin, Sychev, & Sheindlin, 1976).
Extensive properties are related to the intensive ones by the amount of substance present.
Equation of State
“An equation of state of a substance is a relationship among pressure (P), specific volume (v), and temperature (T), and most equations of state are extremely complicated. Therefore, an ideal gas equation is the convenient approximation and it is easily understood and may be used in most of the computational analysis of this book. An ideal gas molecule has no volume or intermolecular forces and most of the gases can be modeled as idea” (Kirillin, Sychev, & Sheindlin, 1976).
PV=NRuT
(2.11)
PV=mRT
(2.12)
PV=RT
(2.13)
PV=ρRT
(2.14)
Where the specific gas constant is related to the universal gas constant Ru = 8315J/Kmol K-1 and the gas molecular weight MW by
R= RuMW
(2.15)
The density ρ is the reciprocal of the specific volume
ρ= 1v= mV.
The conservation of mass principle is a fundamental engineering concept. A description based on mass or weight for a mixture of compounds, existing in either reactant or product state, termed a gravimetric analysis, expresses the total mass in terms of each pure constituent. For a mixture of s total chemical species, a mass fraction mfi for each i component species can be written as:
mfi= mimt
and
∑i=1smfi=1and the total mass m, is then equal to:
∑i=1smi=1
Even though the total mass of a combustion process may remain constant, concentration of constituents such as oxygen or carbon dioxide may change during a reaction. Often, it is more convenient to describe chemically reactive mixtures on a molar basis. For a mixture of s total chemical species, a mole fraction Xi for each species is:
Xi= nint
and
∑i=1sXi=1
Where
nt= ∑i=1sniand mi = ni Mi, where Mi is the molecular weight of species i. For a
mixture of s species, the total molecular weight is:
Mt= ∑i=1sXiMi
The mole, ni is termed a gmole, since it is an amount of substance in grams equal to the molecular weight. The product of mass and local gravitational acceleration is defined as a force. Weight is really correctly used only as a force. Therefore, the mass of a substance remains constant with elevation, but its weight varies with elevation.
2.1.2 First Law of Thermodynamics
First Law – Fixed Mass
The first law of thermodynamics states that the change in the total energy of a closed system of fixed mass and identity is equal to the heat transfer to the system from its surroundings minus the work done by the system on its surroundings; that is, for an infinitesimal change of state,
dE=δQ- δW
(2.21)
The total energy of the system, E, includes the internal energy, U, the kinetic energy, and the potential energy. The energy is a property of the system that is independent of the path taken in going from one state to another. In contrast, the heat transfer,
δQ, and the work transfer,
δW,for any change in the state of the system depend on the manner in which the state of the system is changed. The change in the system energy is described by a total differential, dE. Since the work and heat transfer depend on the path followed by the system, the
δis used to indicate that these increments are not total differentials. For most systems of concern here, the kinetic and potential energy terms can be neglected, so we may express the system energy in terms of the internal energy, that is,
dU= δQ- δW
(2.22)
Integrating over a finite change of state from state I to state2, the first law for a closed system becomes
U2- U1 =Q12- W12
(2.23)
Only rarely in the consideration of combustion processes can we limit ourselves to a fixed mass in a closed system. More generally, the fuel and air enter the combustion zone across certain boundaries, and combustion products are exhausted across other boundaries. It is convenient, therefore, to derive an expression for the change in state of a fixed volume in space, called a control volume, rather than a fixed mass.
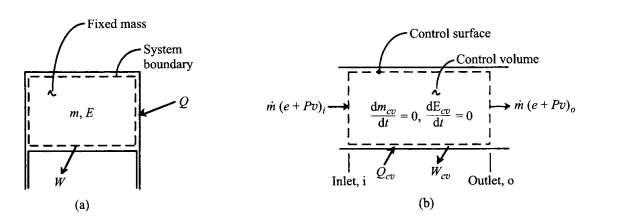
Figure 1(a) schematic of fixed-mass system with moving boundaries above piston (b) control volume with fixed boundaries and steady flow. (Turns, 2000)
First Law – Control Volume
A control volume may be defined in terms of any volume in space in which one has interest for a particular analysis (considering fig. 1(b)). Steady-state, steady-flow form of the first law:
Q̇
CV –
ẆCV =
ṁeo –
ṁei +
ṁ(PoVo – PiVi)
(2.24)
Where
Q̇CV =Rate of heat transferred from surroundings
Ẇ
CV =Rate of work done by control volume excluding flow work
ṁ
eo = Rate of energy flow out of CV
ṁ
ei = Rate of energy flow into CV
ṁ
(PoVo – PiVi) = Net rate of work associated with pressure forces
Main assumptions in the equation:
- The control volume is fixed relative to the coordinate system.
- The properties of the fluid at each point within CV, or on the control surface, do not vary with time.
- Fluid properties are uniform over inlet and outlet areas.
- There is only one inlet and one outlet stream. (Turns, 2000)
Specific energy e of the inlet and outlet stream consist of:
e=u+ 12v2+gz
(2.25)
Enthalpy:
h ≡u+Pv=u+ P ρ
(2.26)
Combining equations 2.24 – 2.26, we have
Q̇
CV –
ẆCV =
ṁ[(ho – hi) –
12(vo2 – vi2)+
g(Zo- Zi)] (2.27)
On a mass-specific basis
qcv- wcv
= (ho – hi) –
12(vo2 – vi2)+
gZo- Zi (2.28)
2.1.3 Stoichiometry
“Stoichiometry is the calculation of relative quantities of reactants and products in chemical reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated” (Wikipedia, https://en.wikipedia.org/wiki/Stoichiometry, 2017).
“Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. The ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry. Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio” (Wikipedia, https://en.wikipedia.org/wiki/Stoichiometry, 2017).
Air-Fuel Ratio
Air–fuel ratio (AFR) is the mass ratio of air to fuel present in a combustion process such as in an internal combustion engine or industrial furnace. The AFR is an important measure for anti-pollution and performance-tuning reasons.
“If exactly enough air is provided to completely burn all of the fuel, the ratio is known as the stoichiometric mixture, often abbreviated to stoich. AFR numbers lower than stoichiometric are considered “rich”. Rich mixtures are less efficient, but may produce more power and burn cooler, which is kinder on the engine. AFR numbers higher than stoichiometric are considered “lean.” Lean mixtures are more efficient but may cause engine damage or premature wear and produce higher levels of nitrogen oxides” (Wikipedia, https://en.wikipedia.org/wiki/Air-fuel_ratio, 2017).
The stoichiometric air-fuel ratio is
(AF)stoic=mairmfuel= 4.76a1MWairMWfuel (2.31)
The equivalence ratio is defined as
∅= (AF)stoic (AF)= FA(FA)stoic (2.32)
∅ >1 :Fuel-rich mixtures
∅ <1 :Fuel-lean mixtures
∅=1 :Stoichiometric mixtures
% Stoichiometric air= 100%∅
(2.33)
% Excess air=1-∅∅100%
(2.34)
2.1.4 Adiabatic Flame Temperature
The temperature of the products in an adiabatic combustion of fuel without applying any shaft work, is defined as the “Adiabatic Flame Temperature”. In a combustion process the heat produced during the exothermic chemical reaction is released to their product and the temperature of the products is raised. There is no possibility for dissipation of the heat to the surrounding and the process will be adiabatic as there is no heat loss to the surrounding.
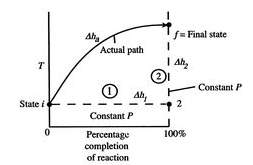
Figure 2 Schematic of adiabatic flame temperature (MIT, n.d.)
As a result, the temperature of the products suddenly increases and it produces a flame. This will heat up the product gases in flame region and the temperature rise will be maximum. This highest temperature is known as the adiabatic flame temperature. The temperature rise depends on the amount of excess air used or the air-fuel ratio. The flame temperature has the highest value for using pure oxygen gas and it decreases by using air. There are two type of adiabatic flame temperature: constant pressure adiabatic flame temperature and constant volume adiabatic flame temperature.
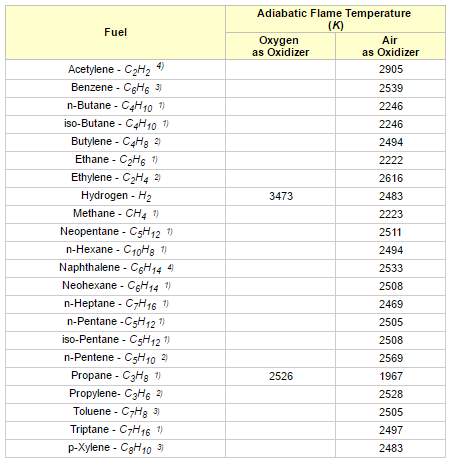
Figure 3 Adiabatic flame temperatures for some common fuel gases (Toolbox, n.d.)
Reactants enter the combustion process at 25oC (77oF) and 1 atm pressure. Products leaves the process at 1 atm pressure. Combustion is stoichiometric without any excess air.
“Excess air will reduce the adiabatic flame temperature and is often introduced to avoid flame temperatures exceeding limits sets by the materials in the combustion system” (Toolbox, n.d.). Methane is the major part in Natural gas.
Constant-Pressure: If a fuel-air mixture burns adiabatically at constant pressure, absolute enthalpy of reactants at the initial state (say, T1 = 298 K, P = 1 atm) equals absolute enthalpy of the products at final state (T = Tad, P = 1 atm).
Hreac (Ti, P) = Hprod (Tad, P) (2.41)
on a per-mass-of-mixture basis,
hreac (Ti, P) = hprod (Tad, P) (2.42)
Constant-Volume
Ureac (Tinit, Pinit) = Uprod (Tad, Pf) (2.43)
where U is the absolute (or standardized) internal energy of the mixture.
Most thermodynamic property compilations and calculations provide H (or h) rather than U (or u). So we consider the fact that:
H = U + P V
Therefore, equation (2.43) becomes
Hreac − Hprod − V (Pinit − Pf) = 0 (2.44)
2.2 Chemical Kinetics
“Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction’s mechanism and transition states, as well as the construction of mathematical models that can describe the characteristics of a chemical reaction” (Wikipedia, https://en.wikipedia.org/wiki/Chemical_kinetics, 2017).
At the macroscopic level, we are interested in amounts reacted, formed, and the rates of their formation. At the molecular or microscopic level, the following considerations must also be made in the discussion of chemical reaction mechanism.
- Molecules or atoms of reactants must collide with each other in chemical reactions.
- The molecules must have sufficient energy (discussed in terms of activation energy) to initiate the reaction.
- In some cases, the orientation of the molecules during the collision must also be considered.
2.2.1 Reaction Rates
Chemical reaction rates are the rates of change in concentrations or amounts of either reactants or products.
Factors that Affect Reaction Rate
Many factors affect rates of chemical reactions, and these are
- Nature of the reactants: Acid-base reactions, formation of salts, and exchange of ions are fast reactions. Reactions in which large molecules are formed or break apart are usually slow. Reactions breaking strong covalent bonds are also slow.
- Physical state: When reactants are in the same phase, as in aqueous solution, thermal motion brings them into contact. However, when they are in different phases, the reaction is limited to the interface between the reactants. Reaction can occur only at their area of contact; in the case of a liquid and a gas, at the surface of the liquid. Vigorous shaking and stirring may be needed to bring the reaction to completion.
- Surface area of solids: In a solid, only those particles that are at the surface can be involved in a reaction. Crushing a solid into smaller parts means that more particles are present at the surface, and the frequency of collisions between these and reactant particles increases, and so reaction occurs more rapidly.
- Concentration: An increase in the concentrations of the reactants will usually result in the corresponding increase in the reaction rate, while a decrease in the concentrations will usually have a reverse effect. The dependences of reaction rates on concentrations are called rate laws. Rate laws are expressions of rates in terms of concentrations of reactants.
- Temperature: Usually, the higher the temperature, the faster the reaction. The temperature effect is discussed in terms of activation energy.
- Catalyst: A catalyst is a substance that alters the rate of a chemical reaction but remains chemically unchanged afterwards. The catalyst increases the rate of the reaction by providing a different reaction mechanism to occur with a lower activation energy.
- Pressure: Increasing the pressure in a gaseous reaction will increase the number of collisions between reactants, increasing the rate of reaction. This is because the activity of a gas is directly proportional to the partial pressure of the gas. This is similar to the effect of increasing the concentration of a solution.
The net rare, Rn, of an elementary reaction is taken as the difference between the forward and reverse rate:
Rn = Forward rate – Reverse rate
At any time, the net rate must be either positive or negative. The higher the concentrations of the reacting molecules, the faster the reaction will proceed. “Since the rate in either direction is proportional to the concentration of each of the reactant molecules, a mass action rate law connects the rate of reaction to the reactant concentrations” (El-Mahallawy & Habik, 2002).
2.2.2 Chemical Equilibrium
“In a reversible reaction, chemical equilibrium is reached when the rates of the forward and reverse reactions are equal and the concentrations of the reactants and products no longer change. This is demonstrated by, for example, the Haber–Bosch process for combining nitrogen and hydrogen to produce ammonia” (Wikipedia, https://en.wikipedia.org/wiki/Chemical_kinetics, 2017).
2.3 Laminar Premixed Flames
“A flame is self-sustaining propagation of a localized combustion zone at subsonic velocities. A flame is said to be localized if it occupies only a small portion of the combustible mixture at a time. The term deflagration defines a discrete combustion wave travelling at subsonic velocities” (Turns, 2000).
A flame is caused by a self-propagating exothermic reaction which is accompanied by a reaction zone and it will propagate through a stationary gas mixture at a characteristic velocity (burning velocity). For most hydrocarbon-air stoichiometric mixtures, this velocity is about 0.4 to 0.6 m/s while for hydrogen-air mixtures, this velocity is several meters per second. The velocity of this wave is controlled by the diffusion of heat and active radicals.
For a flame burning in a mixture of gases of known pressure and composition, two characteristic properties may be defined and measured, the burning velocity and the flame temperature.
Burning velocity is a measure of the rate at which reactants are moving into the flame from a reference point located on the moving frame.
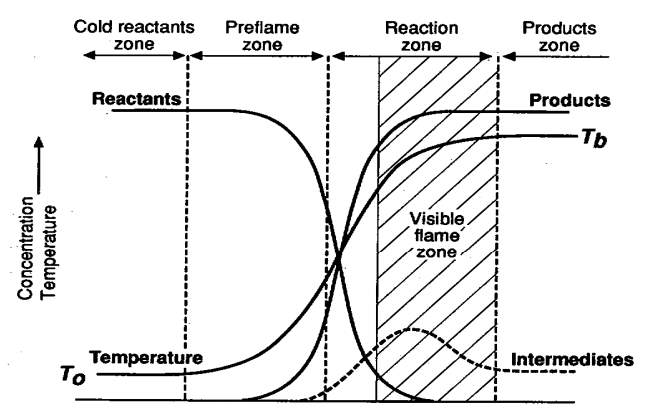
- Figure 4 Laminar flame structure (University of Toronto, n.d.)
Flame temperature can be predicted from thermodynamic data, if we invoke the assumption of chemical equilibrium.
Historically, there have been two approaches to formulating the laminar flame propagation in premixed gases (University of Toronto, n.d.):
1. Thermal propagation: the mixture is heated by conduction to the point where the rate of reaction is sufficiently rapid to become self-propagating.
2. Diffusional propagation: diffusion of active species, such as atoms and radicals, from the reaction zone or the burned gas into the unreacted mixture causes reaction to occur.
Laminar Flame Structure
“Much can be learned by analyzing the structure of a flame. Consider, for example, a flame anchored on top of a single Bunsen burner as shown below. Recall that the fuel gas entering the burner induces air into the tube from its surroundings. As the fuel and air flow up the tube, they mix and, before the top of the tube is reached, the mixture is completely homogeneous. The flow velocity in the tube is considered to be laminar and the velocity across the tube is parabolic in nature. Thus, the flow velocity near the tube wall is very low. This low flow velocity is a major factor, together with heat losses to the burner rim, in stabilizing the flame at the top” (Glassman & Yetter, 2008).
“The dark zone designated is simply the unburned premixed gases before they enter the area of the luminous zone where reaction and heat release take place. The luminous zone is that portion of the reacting zone in which the temperature is the highest; indeed, much of the reaction and heat release take place in this zone” (Glassman & Yetter, 2008).
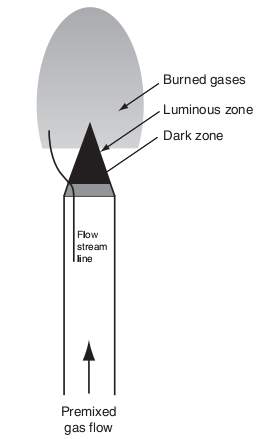
Figure 5 General description of laminar Bunsen burner flame. (Glassman & Yetter, 2008)
Laminar Flame Speed
Burning velocity is also the volume of combustible mixture, at its own temperature and pressure, consumed in unit time by unit area of flame front. It is independent of flame geometry, burner size and flow rate. The burning velocity is essentially a measure of the overall reaction rate in the flame and is important, both in the stabilization of flames and in determining rates of heat release. The burning velocity of a flame is affected by flame radiation, and hence by flame temperature, by local gas properties such as viscosity, thermal conductivity and diffusion coefficient, and by the imposed variables of pressure, temperature, air-fuel ratio and heat of reaction of mole of mixture.
Flammability limits
If small amounts of combustible fuel gas or vapor are added gradually to air, a point will be reached at which the mixture just becomes flammable. A premixed laminar flame will propagate only within a range of mixture strengths:
- Lower limit (lean limit) of flammability, Φ < 1.
- Upper limit (rich limit) of flammability, Φ > 1.
Flammability limits are frequently quoted as percent of fuel by volume in the mixture, or as a percentage of the stoichiometric fuel requirement.
2.3 Laminar Diffusion Flames
When fuel and air enter a combustion system separately, they must mix on a molecular level before reaction can take place. The extent of reaction is strongly influenced by the extent to which that mixing has occurred prior to combustion. This mixing may be achieved solely by molecular diffusion, as in a candle flame, or may be enhanced by turbulence.
“Fuel and air enter in separate streams, diffuse together, and react in a narrow region. While a single value of the equivalence ratio could be used to characterize a premixed flame, the equivalence ratio in the diffusion flame varies locally from zero for pure air to infinity for pure fuel. Combustion in a confined flow may be characterized by an overall equivalence ratio based on the flow rates of fuel and air, but that value may differ dramatically from the value in the flame region” (CaltechAUTHORS).
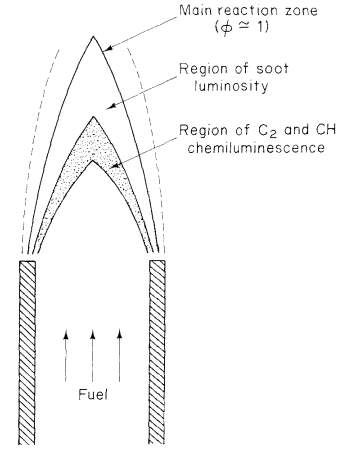
Figure 6 A diffusion flame (CaltechAUTHORS)
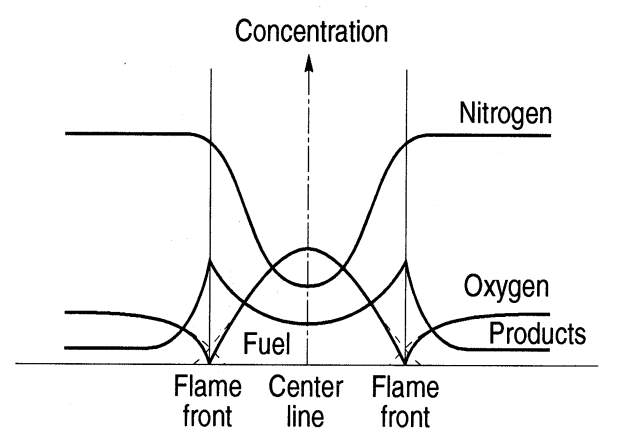
Figure 7 Diffusion Flame Structure (University of Toronto, n.d.)
Emissions
“The fuel used in combustion is carbonaceous, such as gasoline, wood, or coal, and the oxidant is the oxygen in air, although there are non-carbonaceous fuels, notably hydrogen. While such processes are rare, combustion can take place without using air to provide the oxidant. However, combustion can lead to emissions of other compounds due to impurities in the fuel, the presence of nitrogen in air, or incomplete combustion. Specific sources of potentially carcinogenic air pollutant emissions that involve combustion include internal combustion engines (ICEs) (e.g. diesel, gasoline, turbine), external combustion boilers (as used for electricity generation), cement kilns, biomass burning (for cooking, heating, land management, and unplanned fires), waste combustion, and more” (Russell, n.d.).
Combustion-derived air toxics
“A large fraction, but not all, of the air toxics emitted and/or formed during combustion are organic molecules and carbonaceous structures. Some are relatively simple molecules, such as formaldehyde (HCHO), increasing in complexity to compounds like 1,3-butadiene, aromatics, PAHs, and dioxins. Some sources emit soot, which is condensed organic material, part of which may approach elemental carbon” (Russell, n.d.).
Soot Formation
“Soot is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons (Omidvarborna & al, 2015). Soot does not form in premixed flames except when Φ ≥ Φcrit. Conversion of a hydrocarbon fuel with molecules containing a few carbon atoms into a carbonaceous agglomerate containing some millions of carbon atoms in a few milliseconds. Transition from a gaseous to solid phase. Smallest detectable solid particles are about 1.5 nm in diameter (about 2000 amu)” (University of Toronto, n.d.).
“The incandescent soot within the flame is the primary source of diffusion flames’ luminosity. Soot also contributes to radiant heat losses from the flames, with peak emissions at wavelength in the infrared region of the spectrum. It is generally agreed that soot is formed in diffusion flames over a limited range of temperatures, say, 1300k < T < 1600k. The figure below illustrates this point for an ethylene jet flame” (Turns, 2000).
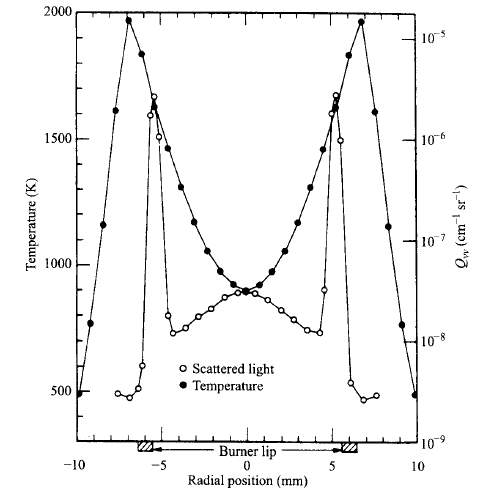
Figure 8 Radial profiles of temperature and scattered light for a laminar ethylene diffusion flame. (Turns, 2000)
Soot formation involves a series of chemical and physical processes (University of Toronto, n.d.):
- Formation and growth of large aromatic hydrocarbon molecules leading to soot inception, i.e, transition to first solid particles (primary particles)
- Surface growth and coagulation of primary particles to agglomerates
- Growth of agglomerates by picking up growth components from the gas phase
- Oxidation of agglomerates
The amount of soot formed in a diffusion flame is strongly dependent on the fuel type. Smoke point is an ASTM standard method to determine sooting tendency of a liquid fuel. The higher the fuel flowrate, the lower is the sooting propensity. Similarly, as with flowrate, the larger the flame height, the lower the sooting propensity.
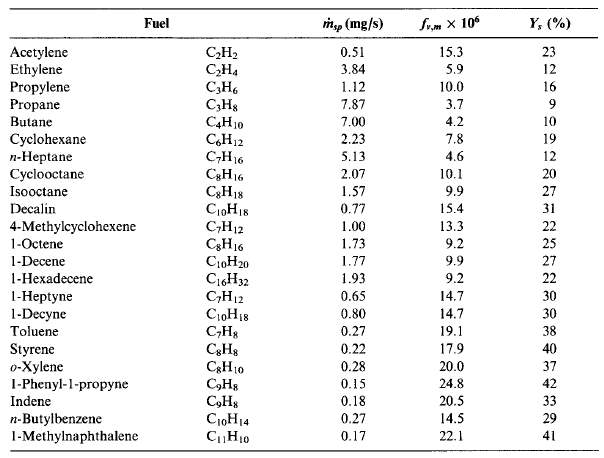
Figure 9 Smoke points, m ̇sp; maximum soot volume fractions, fv,m; and maximum soot yields, Ys; for selected fuels. (Turns, 2000)
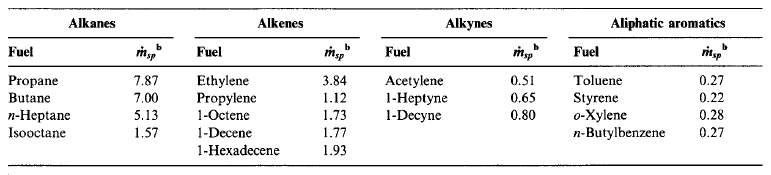
Figure 10 Smoke points by hydrocarbon family (Turns, 2000)
Fuels
A fuel is any material that can be made to react with other substances so that it releases chemical or nuclear energy as heat or to be used for work. The concept was originally applied solely to those materials capable of releasing chemical energy but has since also been applied to other sources of heat energy such as nuclear energy (via nuclear fission and nuclear fusion).
Liquid fuels
Properties of Liquid Fuels
“In combustion process, the fuel can be a liquid, a solid or a gas which is then burnt by a gaseous oxidizer. The knowledge of the fuel properties helps in selecting the right fuel for the right purpose and efficient use of the fuel. The following characteristics, determined by laboratory tests, are generally used for assessing the nature and quality of fuels” (Shaha, 1974):
Density: This is defined as the ratio of the mass of the fuel to the volume of the fuel at a reference temperature of 15°C. Density is measured by an instrument called hydrometer. The knowledge of density is useful for quantity calculations and assessing ignition quality. The unit of density is kg/m3.
Specific gravity: This is defined as the ratio of the weight of a given volume of oil to the weight of the same volume of water at a given temperature. The density of fuel, relative to water, is called specific gravity. The specific gravity of water is defined as 1. Since specific gravity is a ratio, it has no units. The measurement of specific gravity is generally made by a hydrometer.
Viscosity: The viscosity of a fluid is a measure of its internal resistance to flow. Viscosity depends on temperature and decreases as the temperature increases. The measurement of viscosity is made with an instrument called Viscometer. Viscosity is the most important characteristic in the storage and use of fuel oil. It influences the degree of pre-heat required for handling, storage and satisfactory atomization. If the oil is too viscous, it may become difficult to pump, hard to light the burner, and tough to operate. Poor atomization may result in the formation of carbon deposits on the burner tips or on the walls. Therefore, pre-heating is necessary for proper atomization.
Flash Point: The flash point of a fuel is the lowest temperature at which the fuel can be heated so that the vapour gives off flashes momentarily when an open flame is passed over it. Flash point for furnace oil is 66oC.
Pour Point: The pour point of a fuel is the lowest temperature at which it will pour or flow when cooled under prescribed conditions. It is a very rough indication of the lowest temperature at which fuel oil is readily pump-able.
Specific Heat: Specific heat is the amount of kcals needed to raise the temperature of 1 kg of oil by 1oC. The unit of specific heat is kcal/kgoC. It varies from 0.22 to 0.28 depending on the oil specific gravity. The specific heat determines how much steam or electrical energy it takes to heat oil to a desired temperature. Light oils have a low specific heat, whereas heavier oils have a higher specific heat.
Calorific Value: The calorific value is the measurement of heat or energy produced, and is measured either as gross calorific value or net calorific value. The difference being the latent heat of condensation of the water vapour produced during the combustion process.
Sulphur: The amount of sulphur in the fuel oil depends mainly on the source of the crude oil and to a lesser extent on the refining process.
Ash Content: The ash value is related to the inorganic material in the fuel oil. The ash levels of distillate fuels are negligible. Residual fuels have more of the ash-forming constituents. These salts may be compounds of sodium, vanadium, calcium, magnesium, silicon, iron, aluminum, nickel, etc. Typically, the ash value is in the range 0.03-0.07 %. Excessive ash in liquid fuels can cause fouling deposits in the combustion equipment.
Carbon Residue: Carbon residue indicates the tendency of oil to deposit a carbonaceous solid residue on a hot surface, such as a burner or injection nozzle, when its vaporisable constituents evaporate. Residual oil contains carbon residue ranging from1 percent or more.
Water Content: Water content of furnace oil when supplied is normally very low as the product at refinery site is handled hot and maximum limit of 1% is specified in the standard. Water may be present in free or emulsified form.
Types of Liquid Fuels
Biodiesel refers to a family of products made from vegetable oils or animal fats and alcohol, such as methanol or ethanol, called alkyl esters of fatty acids. For these to be considered as viable transportation fuels, they must meet stringent quality standards.
“Biodiesel is commonly produced by the transesterification of the vegetable oil or animal fat feedstock. There are several methods for carrying out this transesterification reaction including the common batch process, supercritical processes, ultrasonic methods, and even microwave methods. Biodiesel can be used in pure form (B100) or may be blended with petroleum diesel at any concentration in most injection pump diesel engines” (Wikipedia, https://en.wikipedia.org/wiki/Biodiesel#Properties, 2017).
CHAPTER 3
METHODOLOGY
Design and Fabrication of a Laminar Diffusion Flame Test Rig
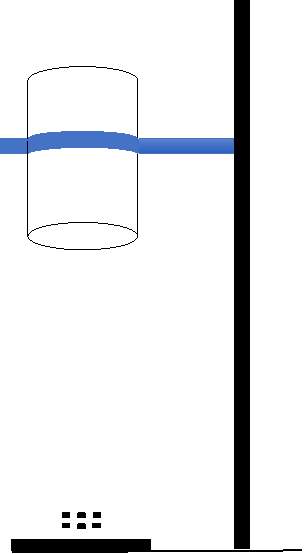
(G) (F)
(A) Container of known graduated volume
(B) Fuel
(C) Wick
(D) Aluminum or mild steel shaft
(E) Fuel inlet
(F) Support stand
(G) Tempered glass beaker
The laminar diffusion flame test rig design consists of container of known graduated volume which holds the fuel, shaft and the wick and the support stand that holds a tempered glass beaker to cover the flame. Different wicks would be used as repeated usage will clog the wick with soot and fuel residue.
Fuels and Fuels Blending for Test
Petro fuel
Petroleum diesel, also called petrodiesel, or fossil diesel is the most common type of diesel fuel. It is produced from the fractional distillation of crude oil between 200 °C (392 °F) and 350 °C (662 °F) at atmospheric pressure, resulting in a mixture of carbon chains that typically contain between 8 and 21 carbon atoms per molecule.
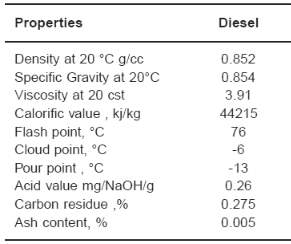
Figure 11 Physical and chemical properties of petrodiesel fuel (Dubey, Sarviya, & Rehman, 2011)
Kerosene is a thin, clear liquid formed from hydrocarbons obtained from the fractional distillation of petroleum between 150 °C and 275 °C, resulting in a mixture with a density of 0.78–0.81 g/cm3 composed of carbon chains that typically contain between 6 and 16 carbon atoms per molecule (Collins, 2007). It is miscible in petroleum solvents but immiscible in water. The American Society for Testing and Materials standard specification D-3699-78 recognizes two grades of kerosene: grades 1-K (less than 0.04% sulfur by weight) and 2-K (0.3% sulfur by weight).
The flash point of kerosene is between 37 and 65 °C (100 and 150 °F), and its autoignition temperature is 220 °C (428 °F). The pour point of kerosene depends on grade, with commercial aviation fuel standardized at −47 °C (−53 °F). Heat of combustion of kerosene is similar to that of diesel fuel; its lower heating value is 43.1 MJ/kg (around 18,500 Btu/lb), and its higher heating value is 46.2 MJ/kg (Annamalai & Puri, 2006).
Biodiesel Fuels
Jatropha
“Jatropha curcas is a species of flowering plant in the spurge family, Euphorbiaceae, that is native to the American tropics, most likely Mexico and Central America” (Jules & Robert, 2008). “Jatropha curcas is a poisonous, semi-evergreen shrub or small tree, reaching a height of 6 m (20 ft)” (Jules & Robert, 2008). It is resistant to a high degree of aridity, allowing it to be grown in deserts. “The seeds contain 27-40% oil” (Achten, et al., 2007) that can be processed to produce a high quality biodiesel fuel, usable in a standard diesel engine.
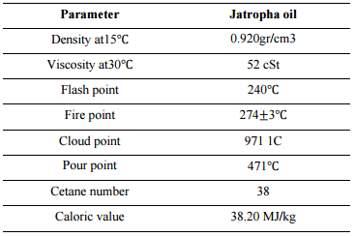
Figure 12 Physical and chemical properties of jatropha oil (Sarker, 2016)
“When jatropha seeds are crushed, the resulting jatropha oil can be processed to produce a high-quality biofuel or biodiesel that can be used in a standard diesel car or further processed into jet fuel, while the residue (press cake) can also be used as biomass feedstock to power electricity plants, used as fertilizer (it contains nitrogen, phosphorus and potassium), or as animal fodder. The cake can also be used as feed in digesters and gasifiers to produce biogas” (Times, 2007). Glycerin is another by-product from Jatropha oil processing that can add value to the crop. Transesterification is a simple chemical reaction that neutralizes the free fatty acids present in any fatty substances in Jatropha.
Neem Seed Oil
Neem (Azadirachta indica) is a tree in the mahogany family Meliaceae which is abundantly grown in varied parts of India. The Neem grows on almost all types of soils including clayey, saline and alkaline conditions. Neem seed obtained from this tree are collected, de-pulped, sun dried and crushed for oil extraction. The seeds have 45% oil which has high potential for the production of biodiesel (Fazal, Haseeb, & Masjuki, 2011).
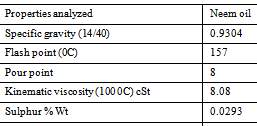
Figure 13 Physical and chemical properties of neem oil (Ademoh, Didam, & Garba, 2016)
It is an evergreen tree related to mahogany, growing in almost every state of India, South-East Asian countries and West Africa (Muñoz-Valenzuela, et al., 2007). It grows in drier areas and in all kinds of soil. It contains several thousands of chemicals which are terpenoids in nature. A mature neem tree produces 30 to 50 kg fruit every year and has a productive life span of 150 to 200 years (Ragit, Mohapatra, Kundu, & Gill, 2011). It has the ability to survive on drought and poor soils at a very hot temperature of 44°C and a low temperature of up to 4°C (Karmakar, Karmakar, & Mukherjee, 2011), and its high oil content of 39.7 to 60% (Martín, et al., 2010).
Palm Kernel Oil
Palm kernel oil is an edible plant oil derived from the kernel of the oil palm Elaeis guineensis(Poku & Kwasi, 2002).It should not be confused with the other two edible oils derived from palm fruits: palm oil, extracted from the pulp of the oil palm fruit, and coconut oil, extracted from the kernel of the coconut (Reeves & Weihrauch, 1979).
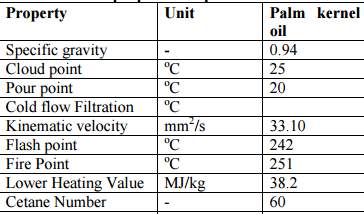
Figure 14 Physical and chemical properties of palm kernel oil (Bello, Oguntuase, Osasona, & Mohammed, 2015)
Chromatographic Analysis of Fuels
As mentioned, the composition of biodiesels can vary from region to region, depending on the source. It is therefore advantageous to this investigation that the composition of the specific biodiesels used in this study is measured, in order to eliminate any discrepancies. The individual emissions characteristics of each fuel can then be related to its composition and perhaps, other attributes. There is a wide range of chemical and physical measurement techniques available for determining the composition of a material, such as photometric analysis, including spectroscopy, electrometric techniques, including electrical conductivity cells and chromatography, including gas chromatography, which uses physical factors. For the analysis of long chain fatty acid esters within the range of 6 to 26 carbons in length, gas chromatography was deemed the most suitable for this work (Hakka, Glaude, Herbinet, & Battin-Leclerc, 2009; Kim, Koo, Cho, & Chun, 2003; Martin, 2013)
Chromatography is a laboratory technique for the separation of a mixture. The mixture is dissolved in a fluid called the mobile phase, which carries it through a structure holding another material called the stationary phase. The various constituents of the mixture travel at different speeds, causing them to separate. The separation is based on differential partitioning between the mobile and stationary phases. Subtle differences in a compound’s partition coefficient result in differential retention on the stationary phase and thus changing the separation (McMurry & John, 2011).
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture (the relative amounts of such components can also be determined). In some situations, GC may help in identifying a compound. In preparative chromatography, GC can be used to prepare pure compounds from a mixture. (Pavia, Lampman, Kritz, & Engel, 2006; Linde, 2012)
References
Achten, W. M., Mathijs, E., Verchot, L., Singh, V. P., Aerts, R., & Muys, B. (2007, November 20). Jatropha biodiesel fueling sustainability? Biofuels, Bioproducts and Biorefining, pp. 283-291.
Ademoh, N. A., Didam, J. H., & Garba, D. K. (2016). Investigation of Neem Seed Oil as an Altanative Metal Cutting Fluid. American Journal of Mechanical Engineering, 191-199.
Annamalai, K., & Puri, I. K. (2006). Combustion Science and Engineering. CRC Press.
Bello, E. I., Oguntuase, B., Osasona, A., & Mohammed, T. I. (2015). CHARACTERIZATION AND ENGINE TESTING OF PALM KERNEL OIL. European Journal of Engineering and Technology, 6.
Britannica.com. (n.d.). (https://www.britannica.com/science/combustion). Retrieved from www.britannica.com: https://www.britannica.com
CaltechAUTHORS. (n.d.). http://authors.library.caltech.edu/25069/4/AirPollution88-Ch2.pdf. Retrieved from authors.library.caltech.edu: http://authors.library.caltech.edu/cgi/oai2
Collins, C. (2007). Implementing Phytoremediation of Petroleum Hydrocarbons. Methods in Biotechnology, 99-108.
ConsumerReports. (2014, April). http://www.consumerreports.org/cro/2012/05/diesel-vs-biodiesel-vs-vegetable-oil/index.htm. Retrieved from www.consumerreports.org: http://www.consumerreports.org/
Dubey, A. K., Sarviya, R., & Rehman, A. (2011). Characterization of Processed Jatropha Oil for use as Engine Fuel. Current World Environment; An International Research Journal of Environmental Science, 101-107.
El-Mahallawy, & Habik. (2002). Fundamentals and Technology of Combustion. Oxford: Elsevier Science Ltd.
Fazal, M., Haseeb, A., & Masjuki, H. (2011). Biodiesel feasibility study: An evaluation of material compatibility; performance; emission and engine durability. Renewable Sustain. Energy Reviews, 1314-1324.
Glassman, & Yetter. ( 2008). Combuustion. Oxford: Elsevier Inc.
Hakka, M. H., Glaude, P.-A., Herbinet, O., & Battin-Leclerc, F. (2009). Experimental study of the oxidation of large surrogates for diesel and biodiesel fuels. Combustion and Flame, pp. 2129 – 2144.
Jules, J., & Robert, E. P. (2008). The Encyclopedia of Fruit & Nuts. CABI.
Karatas, A. E., Intasopa, G., & Gülder, Ö. L. (2013). Sooting behaviour of n-heptane laminar diffusion flames at high pressures . Combustion and Flame, 1650.
Karmakar, A., Karmakar, S., & Mukherjee, S. (2011). Biodiesel production from neem towards feedstock diversification. Indian perspective. Renewable Sustain. Energy Rev., 1050-1060.
Kim, K.-B., Koo, K.-W., Cho, T.-Y., & Chun, H.-G. (2003). Effect of heat treatment on photoluminescence behaviour of BaMgAl10O17:Eu phosphors. Materials Chemistry and Physics, 682 – 689.
Kirillin, V. A., Sychev, V. V., & Sheindlin, A. (1976). Engineering Thermodynamics. Moscow: Mir Publishers.
Kumar, V., & Padam, K. (2013). Study of Physical and Chemical Properties of Biodiesel from Sorghum Oil. Research Journal of Chemical Sciences, Vol. 3(9), 64-68, .
Linde, A. (2012, March 11). Gas Chromatography.
Martín, C., Moure, A., Martín, G., Carrillo, E., Domínguez, H., & Parajó, J. C. (2010). Fractional characterisation of jatropha, neem, moringa, trisperma, castor and candlenut seeds as potential feedstocks for biodiesel production in Cuba. . Biomass Bioenergy, 533-538.
Martin, L. (2013). DEVELOPMENT OF THE GAS PHASE LASER INDUCED PHOSPHORESCENCE TECHNIQUE AND SOOT MEASUREMENTS IN FLAMES USING LASER INDUCED INCANDESCENCE. London.
McMurry, & John. (2011). Organic chemistry: with biological applications (2nd ed.). California: Brooks/Cole Publishing Company.
MIT. (n.d.). http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/node111.html. Retrieved from www.mit.edu: http://web.mit.edu/
Muñoz-Valenzuela, S., Ibarra-López, A. A., Rubio-Silva, L. M., Valdez-Dávila, H., Borboa-Flores, J., Janick, J., & Whipkey, A. (2007). Neem Tree Morphology and Oil Content. In: Issue In New Crops And New Uses. Alexandria,VA: ASHS Press.
Omidvarborna, & al, e. (2015). Recent studies on soot modeling for diesel combustion. Renewable and Sustainable Energy Reviews, 48: 635–647.
Pavia, D. L., Lampman, G. M., Kritz, G. S., & Engel, R. G. (2006). Introduction to Organic Laboratory Techniques (4th Ed.). California: Thomson Brooks Cole Publishing.
Poku, & Kwasi. (2002). “Origin of oil palm”. Small-Scale Palm Oil Processing in Africa. FAO Agricultural Services Bulletin 148, 3.
Ragit, S. S., Mohapatra, S. K., Kundu, K., & Gill, P. (2011). Optimization of neem methyl ester from transesterification process and fuel characterization as a diesel substitute. Biomass Bioenergy, 1138-1144.
Reeves, J. B., & Weihrauch, J. L. (1979). Composition of foods: fats and oils. In S. a. U.S. Dept. of Agriculture, Agriculture handbook 8-4 (p. 4). Washington: Consumer and Food Economics Institute.
Russell, A. (. (n.d.). https://www.iarc.fr/en/publications/books/sp161/161-Chapter4.pdf. Retrieved from www.iarc.fr: http://publications.iarc.fr/
Sarker, K. (2016). Review and Comparison of Various Properties of Jatropha oil Biodiesel. International Journal of Engineering and Technology (IJET), 1966.
Shaha, A. (1974). Combustion Engineering and Fuel Technology. Delhi: Oxford & IBH Publishing Company.
Times, O. (2007, July 28). https://www.thetimes.co.uk/. Retrieved from www.thetimes.co.uk: https://www.thetimes.co.uk/#section-news
Toolbox, T. E. (n.d.). http://www.engineeringtoolbox.com/adiabatic-flame-temperature-d_996.html. Retrieved from www.engineeringtoolbox.com: http://www.engineeringtoolbox.com/
Turns, S. R. (2000). An Introduction to Combustion; concepts and applications. Singapore: The Mc-Graw Hill Companies Inc.
University of Toronto, F. o. (n.d.). http://arrow.utias.utoronto.ca/~ogulder/ClassNotes6.pdf. Retrieved from www.arrow.utias.utoronto.ca: http://arrow.utias.utoronto.ca/
Wikipedia. (2016, December 3 ). https://en.wikipedia.org/wiki/Thermochemistry. Retrieved from www.wikipedia.org: https://en.wikipedia.org/
Wikipedia. (2017, April 2 ). https://en.wikipedia.org/wiki/Air-fuel_ratio. Retrieved from www.wikipedia.org: https://en.wikipedia.org/
Wikipedia. (2017, April 5 ). https://en.wikipedia.org/wiki/Biodiesel#Properties. Retrieved from www.wikipedia.org: https://en.wikipedia.org/
Wikipedia. (2017, March 1). https://en.wikipedia.org/wiki/Chemical_kinetics. Retrieved from www.wikipedia.org: https://en.wikipedia.org/
Wikipedia. (2017, March 29 ). https://en.wikipedia.org/wiki/Diesel_fuel. Retrieved from www.wikipedia.org: https://en.wikipedia.org/
Wikipedia. (2017, March 27). https://en.wikipedia.org/wiki/Stoichiometry. Retrieved from www.wikipedia.org: https://en.wikipedia.org/
Yi, W. (2017, January 10). https://tel.archives-ouvertes.fr/tel-01430861/document. Retrieved from www.tel.archives-ouvertes.fr/tel-01430861: https://tel.archives-ouvertes.fr/tel-01430861
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Engineering"
Engineering is the application of scientific principles and mathematics to designing and building of structures, such as bridges or buildings, roads, machines etc. and includes a range of specialised fields.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




