Hepatoprotective Effect of Alugbati (Basella alba linn) Leaf Aqueous Extract
Info: 7681 words (31 pages) Dissertation
Published: 16th Dec 2019
Tagged: Biology
Hepatoprotective Effect of Alugbati (Basella alba linn) Leaf Aqueous Extract on Isoniazid, Rifampicin, Pyrazinamide- Induced Hepatotoxicity of Female Albino Wistar Rats
Chapter I
Introduction
Background of the Study
Most medications when used in the long term, have hepatotoxic effects. One of the diseases that have a long treatment period is tuberculosis. It is one of the most prevalent infectious diseases worldwide. The Philippines is among the countries that have the highest number of cases of tuberculosis. As part of the Directly Observed Treatment (DOTS), several anti-tuberculosis drugs are prescribed, including Rifampicin, Isoniazid, and Pyrazinamide. These drugs have been shown to cause hepatotoxicity as its adverse effect.
A study done in 2014 in Dawro Zone, Tercha District Hospital Laboratory, South Ethiopia measured liver enzyme levels every 2 weeks for 2 months in 124 TB positive individuals who were under treatment. It was found that 8% of said individuals developed hepatotoxicity.(1) Another study from Qazvin Bouali Hospital had 324 individuals who were TB positive had a drug-induced hepatotoxicity incidence of 4.9% during the seven-year course of the study.(2)The liver plays a crucial role for concentrating and metabolizing many drugs and toxins. The loss of liver function can cause serious consequences to human beings. Thus, it must be protected, especially during long periods of treatment with drugs that are toxic to the liver. One locally available plant that is potentially hepatoprotective is Alugbati (Basella alba L.). It is also known as Ceylon Spinach, Indian Spinach and Malabar Nightshade, is as succulent, branched, smooth, twining and herbaceous vine. It grows well in tropical lowlands and in temperate regions.(3)
A study investigated the effect of ethanolic extract of Basella alba on lead-induced hepatotoxicity in male rats which showed that the levels of alkaline phosphate, aspartate aminotransferase and alanine aminotransferase were significantly reduced while glutathione was significantly increased in Basella alba treated groups compared to lead control group at (p < 0.05). The data suggests that Basella alba reduced lead toxicity. (4)
Another study showed that the aqueous ethanolic extract of aerial parts of Basella alba showed very significant hepatoprotection against both Paracetamol induced and CCl4-induced hepatotoxicity study models in wistar rats. This was evidenced by marked reduction in marker enzymes in serum. Histopathological studies also confirmed the hepatoprotective nature of the extract. (5)
With this, the proponents of this study aim to evaluate the hepatoprotective effect of alugbati among female rats against isoniazid, rifampicin, and pyrazinamide-induced hepatotoxicity.
Objectives of the Study
The main aim of this study is to determine the hepatoprotective effect of alugbati (Basella alba linn) leaf aqueous extract in the liver function test of isoniazid, rifampicin, and pyrazinamide-induced hepatotoxicity on female rats.
Specifically, it aims to:
- Determine the baseline ALT level of the female rats;
- Determine if there is a significant difference between the means of ALT level of the female rats before and after administration of alugbati (Basella alba linn) leaves aqueous extract;
- Determine if there is a significant difference between the means of ALT level of the control groups and the test groups; and
- Determine if there is a significant difference between the means of the ALT level of the different doses used in the different experimental groups.
Significance of the Study
This study will provide experimental evidence on the hepatoprotective effect of alugbati extract on isoniazid, rifampicin, and pyrazinamide-induced hepatotoxicity of female rats. This research is not only useful for specific anti-tuberculosis drugs but can also be applied other similar drugs that can cause liver damage. If proven, this could greatly help certain populations in improving their well-being without spending much as alugbati can easily be grown locally and is inexpensive. To the researchers, this study will also provide additional knowledge on the hepatoprotective effects of alugbati that can be used for further study and drug discovery.
Scope and Limitation
This study is focused on determining the efficacy of alugbati against isoniazid, rifampicin, and pyrazinamide-induced hepatotoxicity. This study will only be limited to the use of healthy, young adult, nulliparous, non-pregnant female rats based on the OECD guidelines for testing of chemicals. Also this study will be focused on the effects of alugbati on only one parameter of the liver function test, ALT. This study will also not be able to perform the Approximate Effective Dose testing due to time constraints. This study will be conducted on January to May of 2017 at the laboratory of Davao Medical School Foundation, Inc.
Definition of Terms
The following terms are defined to give the reader a better comprehension of the terms used in the study:
Tuberculosis. An infection caused by the microorganism Mycobacterium tuberculosis
Alugbati Leaves. It (Basella alba linn) is fleshy, ovate or heart-shaped, stalked, tapering to a pointed tip appearance. Plant to be tested for its effect on liver function test of female rats.
Blood. Specialized body fluid in animals that provide nutrients, oxygen, and waste removal. It is the type of body fluid used as a sample specimen in liver function test.
Enzyme. A protein that act as catalyst in living organism, regulating the chemical reaction. It is one of the analyte used in liver function test.
Extract. A semi-liquid form substance obtained from alugbati leaves.
Hepatotoxicity. Capacity of the drugs or chemical to produce injury to the liver.
Liver. Large lobe glandular organ that has major role in metabolism in the animals, including detoxification.
List of Acronyms
DOTS. Directly Observed Treatment
SGPT. serum glutamic-pyruvic transaminase
ALT. alanine transaminase
ALP. alkaline phosphatase
PZA. Pyrazinamide
INH. isoniazid
RFP. rifampicin
CHAPTER II
Review of Related Literature
Alugbati (Basella alba L. syn. Basella rubra Roxb.)
Alugbati (Basella alba L.) belongs to the family Basellaceae. It is also known as Malabar spinach, Indian spinach, Ceylon spinach, vine spinach, climbing spinach, East-Indian spinach, Chinese spinach, and cyclone spinach. It is a heat tolerant and fast growing perennial vine that has thick tender stems. Its leaves are circular to ovate, thick, rugose, succulent and the color ranges from green to purple. B. alba is native to Southern Asia. It is abundant in countries such as Philippines, Malaysia, Caribbean, South America, and Southeast of Brazil. (6)
The various parts of this plant have been used in the treatment of human diseases and ailments. Traditionally, its leaves have been used to bring sound refreshing sleep when applied on the head. Different preparations of the leaves, sap and roots have also been used to treat acne and ulcers. It has been reported to have antifungal, anticonvulsant, analgesic, anti-inflammatory, anti-diabetic, and androgenic activities.(7)
Phytochemistry
B. alba is a rich source of several vitamins and minerals, including vitamin A, vitamin C, folic acid, thiamine, riboflavin, niacin, calcium, magnesium, and iron. It also contains the essential amino acids arginine, isoleucine, leucine, lysine, threonine, and tryptophan.(8) The plant also has antioxidants like basellasaponins A,B,C, and D, flavonoids, betacyanin, phenolic acid, and ascorbic acid. These antioxidants are able to scavenge reactive oxidant species.(9)
Effect on Hepatotoxicity
Interest on the hepatoprotective effect of B. alba have been recently growing. It was shown in a study by Yanadaiah et al. in 2011, that the aqueous ethanolic extract of B. rubra afforded significant hepatoprotection against Paracetamol- and Carbon tetrachloride-induced hepatotoxicity in wistar rats. The study was conducted using the two popular inducing agents Paracetamol (2mg/kg,p.o.) in 1% CMC and Carbon tetrachloride (2 ml/kg). N-acetyl I-cystine (100mg/kg b.w) and Silymarin (50mg/kg, p.o.) were used as reference drugs in the respective models. The effect was estimated by measuring the enzymatic levels and histopathological studies. The study concluded that the extract gradually reversed the altered values of AST, ALT, and ALP and that it significantly decreased the elevated serum bilirubin towards the normal value. The hepatoprotective effect of the extract was also confirmed through histopathological studies. (10)
Alugbati has been shown to demonstrate hepatoprotective properties comparable to that of silymarin, which is often used as the standard for testing hepatoprotective effects against paracetamol-induced hepatotoxicity. In a study by Das S, et al., six groups of six albino rats each received orally for 6 weeks: vehicle; paracetamol (2 g/kg/day); paracetamol (2 g/kg/day) plus silymarin (50 mg/kg/day); paracetamol (2 g/kg/day) plus B. alba extract (60 mg/kg/day); paracetamol (2 g/kg/day) plus B. alba extract (80 mg/kg/day); and paracetamol (2 g/kg/day) plus B. alba extract (100 mg/kg/day), and the hepatoprotective effect was evaluated by comparing serum bilirubin, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, proteins, alkaline phosphatase and liver histopathology. The results showed that the B. alba leaf extracts had significant hepatoprotective effect in paracetamol-induced hepatotoxicity in albino rats. (11)
Another study shows that the aqueous and ethanolic extract of B. alba had a considerable effect in the bilirubin content of the animals. S. A. Deshmukh and D. K. Gaikwad demonstrated that the B. alba extract showed an increase in bilirubin of the animals. They also found that the extract had increased the like red blood cell count, platelet count, white blood cell count, packed cell volume and hemoglobin concentration while there was a reduction in the activity of liver enzymes like alkaline phosphatase (ALP), alanine aminotransaminase (ALT) and aspartate aminotransaminase (AST). (12)
The ethanolic extract of B. alba leaves were shown to significantly decrease the levels of ALT, AST, and ALP in male albino wistar rats that have been intoxicated with lead. 24 male albino Wister rats were randomly divided into 4 groups. Group 1 was given orally 10ml/kg normal saline while group 2 received 50mg/kg of lead acetate for 4 weeks. Group 3 received 50mg/kg of lead acetate for first week and 250mg/kg Basella alba for 3 weeks. Group 4 received 250mg/kg Basella alba for first week and 50mg/kg of Lead Acetate for the next 3 weeks. Then, levels of the liver enzymes in the serum were measured. The results showed that the levels of alkaline phosphate, aspartate aminotransferase and alanine aminotransferase were significantly reduced while glutathione was significantly increased in Basella alba treated groups compared to lead control Group at (p < 0.05). The data suggests that Basella alba reduces lead toxicity. The hepatic protection was attributed to the basellasaponins in the plant. (13)
Basellasaponins
One of the unique components of B. alba causing the hepatoprotective effect are the basellasaponins. They belong to a class of organic compounds known as triterpenesaponins which are synthesized by plants as part of their development. These are glycosylated derivatives of triterpenesapogenins where the sapogenin moiety backbone may be based on the oleanane, ursane, taraxastane, bauerane, lanostane, lupeol, lupane, dammarane, cycloartane, friedelane, hopane, 9b,19-cyclo-lanostane, cycloartane, or cycloartanol skeleton. (14)
Saponins have very low toxicity when administered in mammals and the saponins naturally presents in foods are generally non-toxic. (15)It has been found that one of the bioactivities of saponins is hepatic protection. (16)They have been found to have this effect in various plants Specifically, the basellasaponins found in B. alba are antioxidants that scavenge reactive oxidant species. (17)
Toxicity
In Alugbati (Basella alba linn) extract toxicity studies, there were no behavioral changes up to 4 hours into albino rats. No mortality was observed up to the end of 48hours even at the maximum tested dose level of 2000mg/kg per oral as a result an effective dose of 250mg/kg. (18)
Pyrazinamide-induced hepatotoxicity
Drug-induced hepatotoxicity of Pyrazinamide reported at dose of 40-70mg/kg for 2 months showed an increase of liver enzymes, and shows hepatitis symptoms. (19)Pyrazinamide, an anti-tuberculosis drug was administered at 500 mg/kg/day over 7 weeks proved to induce Liver Injury. Enzymatic testing revealed an elevated AST and ALT in rats.(20)
Pyrazinamide in a minimum dose of 250 mg/kg in rats, showed an elevated amount of bilirubin;and maximum dose of 1000 mg/kg/day body weight has significant increase in ALT and AST activities for 45 days in rats. Liver tissue of rats displayed prominent signs of fibrosis development. (21)
Pyrazinamide was also found to have caused drug-induced hepatotoxicity at dose of 1.0 and 2.0 g/kg-1 by gavage for 1 month. There is decrease weight of rats; pyrazinamide was proven to produce oxidative stress and metabolic disturbance in protein synthesis which primarily observed in the Liver. (22)
Isoniazid and Rifampicin as Hepatotoxic Agents
Rats were treated with INH alone (100 mg/kg) or co-administered with RFP (100 mg/kg, ig) for 10 days and 21 days. For a 10days of INH-treatment, liver impairment appeared after 21 days treatment, However, in INH-RFP group for 10 days, similarly, hepatic injury is equal to INH group appeared after 21 days. (23)
Normal mice injected with Isoniazid and Rifampicin (INH/RIF) in saline at doses of 50/100 mg/kg once daily at a volume of 10 mL/kg for 3 weeks induced hepatotoxicity. GSP analysis was performed 16 hours after treatment at 0, 2, and 3 weeks to quantify residual liver function. Plasma enzyme activities (AST and ALT) were determined. The mice showed significantly abnormal serum levels of AST and ALT, and GSP value. (24)
In the study, “Protective Effects of Quercetin against Isoniazid and Rifampicin Induced Hepatotoxicity in Rats,” the effects of long-term administration of anti-tubercular drugs on the serum levels of liver enzymes of rats treated with a combination of Isoniazid (INH) and Rifampicin (RFP) had a highly significant increase in serum ALT activity in 42 days. A section of liver tissue from treated rats showed liver toxic injury with mild degenerative changes in addition to focal lobular inflammation, eosinophils infiltration in the portal tracts and lobular parenchyma, with marked Kupffer cell hyperplasia and scattered apoptotic hepatocytes. (25)
Anti-tuberculosis drugs (Isoniazid, Rifampicin and Pyrazinamide) as Hepatotoxic Agents
The animals in a study entitled “Silymarin protects liver against toxic effects of anti-tuberculosis drugs in experimental animals” received 350 mg/kg of Pyrazinamide (PZA) via intra gastric route and intra-peritoneally injected with Isoniazid (INH) (50 mg/kg) and Rifampicin (RIF) (100 mg/kg). These drugs were given once daily over 14 days. Liver enzymes test: ALT, AST and ALP were measured before and after treatment. INH + RIF + PZA in group IV of the experimental animals caused a 2-fold increase (P < 0.001) from their baseline of ALT. The activities of serum AST and ALP were also increased (P < 0.001) approximately 75% which indicates that the drugs had altered hepatocellular integrity. However, in this study, Pyrazinamide did not seem to exacerbate the hepatotoxic effects of INH and RIF.(26)
Ursodeoxycholic Acid and its Hepatoprotective Activity
Ursodeoxycholic acid was administered to patients with chronic active hepatitis in three different doses (250mg, 500mg and 750 mg) daily for 2months. A significant decrease in serum transaminases and gamma-glutamyl transpeptidase were observed when taking the least dose and with the same effect when higher doses were given. There was no significant effect on bile acid metabolism during ursodeoxycholic acid therapy.(27)
Ursodeoxycholic acid (a hydrophilic bile acid that occurs naturally in human bile in a small quantity) showed a reduction of biochemical markers of both cholestasis as well as hepatocellular damage in patients suffering from chronic liver diseases. Evidences suggest that it is most beneficial in patients who has primary biliary cirrhosis, cholestasis in relation to cystic fibrosis and primary sclerosing cholangitis. Improvements in serum transaminase, gamma glutamyltransferase and alkaline phosphatase was noted; an enzyme glutamine dehydrogenase ( specific indicator of hepatocyte damage) was also decreased. Dosage was given around 10mg/kg/day for sclerosing cholangitis and biliary cirrhosis with caution when giving to biliary cirrhosis at stages 3 and 4.(28)
Theoretical Framework
Alugbati (Basella alba linn) contains basellasaponins which are responsible for its ability to decrease the levels of serum AST, ALT, ALP, and bilirubin in rats experimentally induced with hepatic toxicity. These basellasaponins are antioxidants that scavenge reactive oxygen species (ROS) that are normally produced by metabolism of normal cells. However, in disease processes of the liver and inflammatory reactions, redox is increased as a way of phagocytes to kill offending agents. This process usually involves mitochondrial dysfunction through an intracellular oxidant stress in hepatocytes leading to oncotic necrosis and less apoptosis. Additionally, the release of cell contents contributes to the inflammatory injury. (29) Several drugs, including isoniazid, rifampicin, and pyrazinamide can produce oxidative stress in the liver.
Basellasaponins in alugbati, being an antioxidant inhibit the activity of ROS. They scavenge or dispose of these free radicals. As a saponin, it is hypothesized that they protect the physicochemical properties of the cell membrane from ROS-induced cellular dysfunction. They also help inhibit the formation of ROS. (30)
Conceptual Framework



Research Hypothesis
Null Hypotheses
- There is no significant difference between the serum ALT levels of isoniazid, rifampicin, and pyrazinamide-induced hepatotoxicity female rats before and after taking the alugbati (Basella alba linn) leaf aqueous extract.
- There is no significant difference between the serum ALT levels of the control groups and the test groups.
- There is no significant difference between the serum ALT levels of the different experimental dose groups.
Chapter III
Methodology
Research Design
This study will utilize a 5-group pre-test and post-test experimental research design. In this study design, participants are studied before and after treatment is deliberately introduced. Manipulation and control of the variables as well as random selection and assignment of the samples will be involved.
Research Locale
The study will be conducted in Davao Medical School Foundation, Incorporated.
Sources of Data
The data shall be obtained by the investigators from the observed changes in the serum ALT levels of the rats before and after undergoing alugbati (Basella alba linn) extract treatment. An autopsy will also be done for histopathologic examination.
Data Gathering Instrument
Blood samples collected from the rats shall be sent to a legitimate laboratory that conducts blood chemistry assays for animals. The ALT levels of the rats will preferably be tested using well-calibrated automated machines to ensure data reliability and accuracy. Liver samples will be sent to a laboratory for preservation to be studied by a pathologist.
Sampling Technique
The selection of rats will be based on the OECD Guidelines for testing chemicals. Healthy, young adult, nulliparous, and non-pregnant female albino wistar rats will be employed. The rationale is that although there is little difference between male and female rats in terms of sensitivity to LD50 tests, the females are generally more sensitive when differences are observed. The rats are to be randomly selected and assigned to a group.(31)
The experimentation proper will be comprised of 36 female rats. At the beginning of the study, they will be labeled for identification and shall be assigned to a group randomly. They shall be kept in the cages assigned to their treatment group. (32)
Preparation of Extracts
In preparation of alugbati leaf extract, the alugbati leaves will be washed first with distilled water to remove extraneous materials such as dusts, debris, and associated insects. Using a clean mortar and pestle, the leaves will be grounded and placed in an Erlenmeyer flask with water as the extracting solvent(33)will be stored for 24 hours. After such period, the mixture will be filtered using filter paper and the filtrate will be poured into a 250 ml beaker. The filtrate will then be poured into the rotating flask of the rotavapor and will be subjected to drying at 40 degrees Celsius. The residue will then be transferred into a pre-sterilized 50 ml beaker covered with autoclavable plastic and aluminum foil. Finally, they will be placed in pre sterilized vials to ensure unexposed extracts. This will be carried out by storing them in the refrigerator. (34)















Figure 1. Rotary Evaporation Process
Acute Toxicity Testing
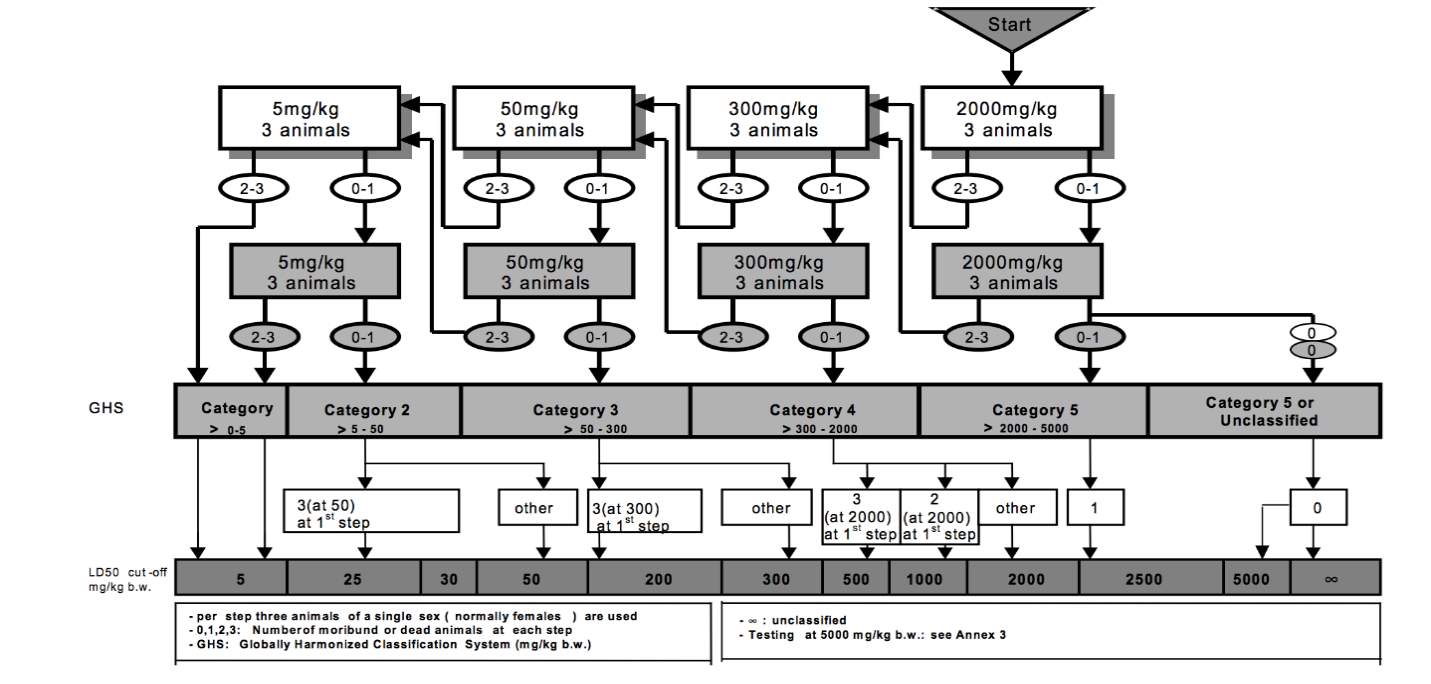
Acute toxicity testing will be done using the OECD Guidelines for Testing of Chemicals (OECD 423). The test will classify the aqueous extract of Alugbati (Basella alba linn) according to the Globally Harmonized System for the classification of chemicals which cause acute toxicity. This will follow a stepwise procedure outlined in Appendix 1.
Selection of Animal Species
Healthy young adult (aged 8 to 12 weeks old), weighing 150 to 250g, nulliparous, and non-pregnant female albino wistar rats will be used. Their weight should fall in an interval within
±20% of the mean weight of any previously dosed animals at the beginning of testing. They shall have been housed and fed in the following conditions: temperature of 22oC; humidity of at least 30% not exceeding 70%; cycled for 12 hours light, 12 hours dark; and with conventional laboratory diets and unlimited supply of drinking water. They shall be allowed to acclimatize to the laboratory setting for at least 5 days prior to testing. They shall also be randomly selected and marked for identification.
Procedure
There will be 5 groups in this study with 6 randomly chosen rats in each group: group I, a negative control group with Isoniazid, Rifampicin, Pyrazinamide (I/R/P) induction; group II, a positive control group with I/R/P induction, plus ursodeoxycholic acid (80 mg/kg) (35); group III, with I/R/P induction plus alugbati extract (125 mg/kg); group IV, with I/R/P induction plus alugbati extract (200 mg/kg); group V, with I/R/P induction plus alugbati extract (500 mg/kg); (36). For the pre-test: 0.5 ml of blood sample from each rat will be extracted from the tail vein for the determination of the baseline alanine aminotransferase (ALT). Simultaneously, all treatments and hepatotoxic agents (I/R/P) will be given daily for 35 days. For groups II to VI, the administration of Basella alba extract and ursodeoxycholic acid will be given 45 minutes prior to induction of I/R/P. (37)The doses that will be used for hepatotoxic agents are: Isoniazid (7.5mg/kg), Rifampicin (10mg/kg), Pyrazinamide (35mg/kg). The doses of the alugbati extracts are based on the results of the Acute Toxicity Test prior to the experiment proper, and the doses used for AED test as shown in index 2. The rats will be kept in cages with a total floor area of 174 in2 and a height of 7 in2 under standard condition. After administration of last dose, the animals will be given a rest overnight. For the post-test: 0.5 ml of blood will be drawn from the tail vein of each rat to analyze their ALT levels. A representative rat from each of the 6 groups will be sacrificed and autopsied for histopathological study of their liver. The randomly selected rat shall be humanely killed through cervical dislocation by a skilled personnel. A highly capable person shall also collect the liver of the rats and place it in a preservative. The samples will then be sent to a pathologist.(38)
If any deaths shall occur during the course of the study, they shall be accounted for as an endpoint. Blood samples shall still be collected for serum ALT determination and their livers will be sent to a pathologist for histopathological study to observe for changes attributable to hepatotoxicity.
Statistical Treatment
Data will be analyzed through one-way ANOVA followed by Bonferoni’s test to determine the variability between groups. A p-value of <0.05 will be the level of significance. (39)
Ethical Considerations
- Responsibility must be assumed for the conduct of an animal experiment throughout the entire duration of the experiment which involves the following phases: definition of objectives, selection of animals (species, breed, strain), ethical balancing, test plan, application for authorization, acquisition and keeping of the animals, preparation of the animals for the intervention and treatment; conduct of the experiment, intervention and treatment, monitoring of the animals, documentation of all intervention and treatment, measurements and observations; conclusion of the final experiment followed by restoration of the welfare of the animals or killing of the animals; evaluation of the research findings, publication, reporting to the relevant authorities.
- The ethical approach of respect for life requires that the maximum gain in knowledge is achieved using the minimum possible number of laboratory animals and the limitation of their suffering to the essential minimum. If the suffering of individual animals can be reduced significantly through the use of a larger number of animals, the reduction of individual suffering shall take priority over the reduction of the number of animals used in the experiment.
- All persons involved in animal experiments are obliged to support the welfare and minimum possible suffering of the laboratory animal.
- Proper planning should be practiced and appropriate measures be performed in handling animals. Such measures should be approved by the authorized personnel and clinical professionals manning the area.
- The following hazard controls should be observed and practiced while handling animal subjects:
- Engineering controls
- Personal Protective Equipment (PPE)
- Medical Screening
- Safe Practices
- Instituional Programs
Moreover, training on handling techniques should be conducted before the actual handling of animals subjects.
- There should be a pest control program that is scheduled regularly. This should be done by a certified professional. With regards to the housing area, it should be designed in a way that entry of insects and vermin are prevented. The institution’s standard procedure and protocol for pest control shall be sufficient, if there is such. If possible, the usage and practice of nontoxic means of pest control shall be utilized. However, if need be, toxic chemicals should be utilized at a minimal level and the usage shall be documented, and with the coordination of the animal management staff.
- Methods should be humane should traps be utilized; observation and humane euthanasia shall be practiced should pests be caught alive.
- Animal biosecurity practices should be applied to all species, but they are most important when housing large numbers of animals in intensive housing conditions.
- There will be separation of clean and soiled caging and equipment. The practices that reduce the likelihood of cross contamination if an infectious agent is inadvertently introduced.
- Aseptic technique is used to reduce microbial contamination to the lowest possible practical level.
- The procedures to ensure that all biologics administered to animals are free of contamination; and procedures for intra- and interfacility animal transport.
- Experiments on animals shall be carried out in accordance with the latest developments. Known prophylactic, diagnostic and therapeutic processes shall be taken into account and the scientific guidelines provided by international expert bodies shall be observed.
- If pain, suffering or stress are inevitable concomitants of an experiment, their duration and intensity must be limited to the minimum. To this end, the animals shall be monitored by specially trained personnel in accordance with predefined criteria and at predefined times and measures necessary to alleviate suffering shall be taken insofar as this is compatible with the objective of the experiment. The animal must be able to express its sensations and where possible avoid painful stimuli. Hence, the use of substances that induce paralysis without loss of consciousness and analgesic effects is unauthorized.
- The procedures done in this study do not necessitate the infliction of injury or pain to the rats. However, in case the rats would show signs of pain or other adverse effects due to the treatment schedules, the attention of a veterinarian will be called upon to alleviate their suffering.
- In all experiments that give rise to long or chronic suffering or necessitate repeated intervention, all possible measures must be undertaken to alleviate suffering and dispel fear and anxiety. The professional care of the animals, before, during and after the experiment are particularly important in this context.
- Continuous physical restraint may only be resorted to if other processes have been considered and deemed unsuitable. All possible measures must be taken to alleviate fear and anxiety, in particular the careful and protective familiarization of the animal with the test conditions.
- If distressing measures, such as the restriction of food or water or the withholding of other important environmental factors or administration of pain stimuli are unavoidable, they must be recorded in detail in the test protocol. To ensure that the distress caused does not exceed an acceptable level, the effects of these measures on the animal shall be monitored through the collection of the relevant data.
- Pyrazinamide is known to increase the levels of AST and ALT in rat subjects and can cause fibrosis at certain dosages. A study with regards to the toxicity of ethanolic extract of B. alba leaves at 2000 mg/kg was conducted, no toxicity was noted on the study. To avoid unnecessary suffering, clearly defined termination criteria must be established for all animal experiments. Animals which experience serious suffering must be killed as quickly as possible using a pain-free method.
- The animals for the study shall be purchased from a local breeder, the Philippine Institute of Traditional and Alternative Health Care (PITAHC). Upon transport, careful moving of the containers holding animals should be observed. It should be handled gently. Moreover, it should be kept away from excessive noise or vibration, and be maintained as level as possible to avoid toppling of the animals.
- Laboratory animals should be sheltered and cared for in accordance with the principles of proper animal guardianship. Feeding and housing is handled by animal experts from Philippine Institute of Traditional and Alternative Health Care (PITAHC) to ensure constant environmental conditions to all rats and avoid unnecessary stressors that can affect the result of the study. Every effort must be made to ensure that pens and cages are well made and generously sized and that the animals have adequate opportunities for activity and social contact.
- The primary enclosure of the rats shall allow for their normal physiologic and behavioral needs. It shall also allow for social interaction and hierarchy. It shall be clean and dry, well-ventilated, secure, and free of sharp edges that could injure the animals.
- Whether and individual death or during significant mortality events, the institution’s policy and protocol on disposal of the carcasses of laboratory animals shall be followed.
- Animals must be euthanized by trained personnel using the proper technique, equipment, and agents. The method should utilize minimal restraint, and must be appropriate for the age and species of the animals.
- The surviving rats shall be endorsed to the Philippine Institute of Traditional and Alternative Health Care.
Bibliography
1. Incidence of antituberculosis-drug-induced hepatotoxicity and associated risk factors among tuberculosis patients in Dawro Zone, South Ethiopia: A cohort study . Wondwosen, Abera, Waqtola, Cheneke and Gemeda, Abebe. 1, South Ethiopia : Elsevier, March 2016, Vol. 5.
2. Drug-induced Hepatitis (Abundance and Outcome During Course of Tuberculosis Treatment): Seven Year Study on 324 patients with Positive Sputum in Iran. Bhargi, G. and Samimi, R. 2, Qazviz, Iran : s.n., 2011, Vol. 16.
3. Cagayan De Oro Government. Cagayan De Oro.Da.Gov. [Online] [Cited: December 11, 2016.] http://cagayandeoro.da.gov.ph/wp-content/uploads/2013/04/ALUGBATI.pdf.
4. Bamidele, O. and Adebiyi, T.I. The Effect of Ethanolic Extract of Basella Alba L. Leaves on Lead Induced Hepatotoxicity in Male Albino Wistar Rats (Rattus Norvegicus). [Online] 2015.
5. HEPATOPROTECTIVE ACTIVITY OF AQUEOUS ETHANOLIC EXTRACT OF AERIAL PARTS OF. Yandaiah, J.P. and Lakshmi, Mohana S. 5, Andhra Pradesh, India : International Journal of Pharmacy and Pharmaceutical Sciences, 2011, Vol. 3.
6. A review of the taxonomy, ethnobotany, phytochemistry and Pharmacology of Basella Alba (Basellacea). Deshmuk, S.A. and Gaikwad, D.K. 1, Maharashtra, India : Journal of Applied Pharmaceutical Science, January 2014, Vol. 4.
7. A Review on the Medical Importance of Basella Alba L. Adhikari, R., Kumar, N. and Shruti, S.D. 2, Karnataka, India : International Journal of Pharamaceutical Science and Drug Research, 2012, Vol. 4.
8. A Review on Taxonomy, ehtnobotany, phytochemistry and pharmacology of Basella Alba (Basellaceae). Deshmukh, S.L and Gaikwad, D.K. 1, Maharashtra, India : Journal of Apllied Pharmaceutical Science, 2014, Vol. 4.
9. The effect of ethanolic extract of Basella alba l. Leaves on lead induced hepatotoxicity in male albino wistar rats (rattus norvegicus). Bamidele, O., Adibele, T. and al, et. 5, Osun State, Nigeria : American Institute of Science, 2015, Vol. 1.
10. Hepatoprotective Activity of Aqueous Ethanolic Extract of Basella Alba Linn Against Carbon Tetrachloride and Paracetamol-Induced Hepatotoxicity in Rats. 5, Anhdra Pradesh, India : International Journal of Pharmacy and Pharmaceutical Sciences, 2011, Vol. 3.
11. Evaluation of Hepatoprotective Activity of aqueous extracts of leaves of Basella Alba in Albino Rats. Researchgate. [Online] Taylor and Francis, November 18, 2014. [Cited: December 11, 2016.] https://www.researchgate.net/publication/267744990_Evaluation_of_hepatoprotective_activity_of_aqueous_extracts_of_leaves_of_Basella_alba_in_albino_rats).
12. A Review of the taxonomy, ethnobotany, phytochemistry and pharmacology of Basella Alba (Basellaceae). 1, Maharashtra, India : Journal of Pharmaceutical Science, 2014, Vol. 4.
13. The Effect of Ethanolic Extract of Basella Alba L. Leaves on Lead Induced Hepatotoxicity in Male Albino Wistar Rats ( Rattus Norvegicus). Bamidele, O. and Adibeye, T.I. 5, Osun State, Nigeria : International Journal of Plant Science and Ecology, 2015, Vol. 1.
14. Metabolic Card for Basellasaponin D. The Metabolomics Innovation Centre. [Online] [Cited: December 11, 2016.] http://www.hmdb.ca/metabolites/HMDB39402.
15. Triterpenoid Saponins. Garai, S. 6, s.l. : National Products Chemistry and Research, 2014, Vol. 2.
16. Phytochemistry and bioactivity of tripertene Saponins from Amaranthaceae . Springerlink. [Online] August 2014. [Cited: December 11, 2016.] http://link.springer.com/article/10.1007/s11101-015-9394-4.
17. The Effect of Ethanolic Extract of Basella Alba l Leaves on Lead induced hepatotoxicity in male albino wistar rats (Rattys Norvegicus). Researchgate. [Online] August 2015. [Cited: December 11, 2016.] https://www.researchgate.net/publication/280880482_The_effect_of_ethanolic_extract_of_Basella_alba_l_Leaves_on_lead_induced_hepatotoxicity_in_male_albino_wistar_rats_rattus_norvegicus.
18. Investigation on Anti inflammatory property of Basella Alba linn Leaf Extract. Roda, R. and Kota, A. et al. 1, Prakurthi Nagar, India : International Journal of Pharmacy and Pharmaceutical Sciences, 2012, Vol. 4.
19. A Novel Mechanism Underlies the Hepatotoxicity of Pyrazinamide. Shih, T. and Pai, C. s.l. : American Society for Mirobiology, 2013.
20. A Novel Mechanism underlies the Hepatotoxicity of Pyrazinamide. Shih, T. and Pai, C. 4, s.l. : Antimicrobial Agents and Chemotherapy, 2013, Vol. 57.
21. Epigenetic Changes in the Rat Livers induced by Pyrazinamide Treatment. Kovalenko, B.M. and Bagnyukova, T.V. Jefferson, AR, USA : Elsevier, 2007, Vol. 225.
22. Pyrazinamide induced hepatotoxicity and gender differences in rats as revealed by a H NMR based metabolomics approach. Zhao, H. and Si, Z. s.l. : Journal of British Toxicology Society and The Chinese Society of Toxicology, 2016.
23. CYP2E1 mediated Isoniazid induced hepatotoxicity in Rats. PubMed. [Online] May 25, 2004. [Cited: December 11, 2016.] https://www.ncbi.nlm.nih.gov/pubmed/15132840.
24. Protective Effects of Kaempferol on Isoniazid and Rifampicin Induced Hepatotoxicity. PubMed. [Online] April 17, 2013. [Cited: December 11, 2016.] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3691431/.
25. [Online] http://pubs.sciepub.com/ajps/2/3/3/.
26. Silymarin protects liver against toxic effects of anti tuberculosis drugs in experimental animals. Springerlink. [Online] July 5, 2008. [Cited: December 11, 2016.] http://link.springer.com/article/10.1186/1743-7075-5-18.
27. Effects of Ursodeoxycholic Acid on serum liver enzymes and bile acid metabolism in chronic active hepatitis: a dose response study. PubMed. [Online] February 13, 1991. [Cited: December 11, 2016.] https://www.ncbi.nlm.nih.gov/pubmed/1671665.
28. Ursodeoxycholic Acid in Chronic liver disease. GUT BMJ. [Online] [Cited: December 11, 2016.] http://gut.bmj.com/content/32/9/1061.full.pdf.
29. Jaeschke, H. Rective Oxygen and mechanisms of inflammatory liver injury: Present concepts. Researchgate. [Online] January 26, 2011. [Cited: December 11, 2016.] https://www.ncbi.nlm.nih.gov/pubmed/21199529.
30. Antioxidant and Antibacterial activities of Saponin functions of Erythropheleum suaveolens (Guill. and Peri) stem bark extract. Akinpelu, B.A. and Igbeneghu, O.A. 18, Ibadan, Nigeria : Academic Journals, 2014, Vol. 9.
31. OECD Guideline for Testing of Chemicals. OECD.org. [Online] December 17, 2001. [Cited: December 11, 2016.] http://www.oecd.org/chemicalsafety/risk-assessment/1948362.pdf.
32. OECD Guidelines for Testing of Chemicals. OECD.org. [Online] December 17, 2011. [Cited: December 11, 2016.] http://www.oecd.org/chemicalsafety/risk-assessment/1948362.pdf.
33. OECD Guidline for Testing of Chemicals. OECD.org. [Online] December 17, 2001. [Cited: December 11, 2016.] http://www.oecd.org/chemicalsafety/risk-assessment/1948362.pdf.
34. Antifungal Property of Ethanol Extract of Carica papaya Leaves Against Malassezia Species. al., Rivas et. Iligan City : s.n., 2012.
35. Ursodeoxycholic acid dose-dependently improves liver injury in rats fed a methionine and choline deficient diet. Hepatology Research. [Online] June 28, 2011. [Cited: December 11, 2016.] http://onlinelibrary.wiley.com/doi/10.1111/j.1872-034X.2011.00820.x/full.
36. Evaluation of Hepatoprotective Activity of aqueous extracts of leaves of Basella Alba in Albino Rats. Researchgate. [Online] Taylor and Francis, November 18, 2014. [Cited: December 11, 2016.] https://www.researchgate.net/publication/267744990_Evaluation_of_hepatoprotective_activity_of_aqueous_extracts_of_leaves_of_Basella_alba_in_albino_rats).
37. Hepatoprotective Activity of vitex Negundo leaf extract against Anti tubercular drugs induced hepatotoxicity. Science Direct. [Online] 2008. [Cited: December 11, 2016.] http://www.sciencedirect.com/science/article/pii/S0367326X08001305.
38. Ursodeoxycholic dose dependently improves liver injury in rats fed a methionine and choline deficient diet. Hepatology Research. [Online] June 28, 2011. [Cited: December 11, 2016.] http://onlinelibrary.wiley.com/doi/10.1111/j.1872-034X.2011.00820.x/full.
39. Hepatoprotective activity of Ocimum Sanctum alcoholic leaf extract against paracetamol induced liver damage in Albino Rats. US National Library of Medicine National Institutes of Health. [Online] March 2011. [Cited: December 11, 2016.] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3119265/.
APPENDIX 1
Test Procedure with a Starting Dose of 2000 mg/kg body weight
APPENDIX 2
Computation for Approximate Effective Dose
Dose#1: 3.98mg/kg X antilog0.6 = 15.84mg/kg
Dose#2: 15.84mg/kg X antilog0.6 =63.10mg/kg
Dose#3 63.10mg/kg X antilog0.6 = 251.20mg/kg
Dose#4 251.20mg/kg X antilog0.6 = 1000mg/kg
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Biology"
Biology is the scientific study of the natural processes of living organisms or life in all its forms. including origin, growth, reproduction, structure, and behaviour and encompasses numerous fields such as botany, zoology, mycology, and microbiology.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




