Evaluating Elasticity of Soft Tissue using Surface Acoustic Wave (SAW)
Info: 9194 words (37 pages) Dissertation
Published: 21st Feb 2022
Tagged: Medical Technology
ABSTRACT
Investigating the elasticity in human skin and the mechanical properties of soft tissue by using surface acoustic waves (SAW) is important in diagnostic medical applications. This dissertation benefits from two sets of experiments:
The first set includes five experiments simulated and processed by a Finite Element Analysis software (ANSYS: APDL). The surface acoustic waves (SAW) is generated by a laser pulse and the SAW results are then processed to get the phase velocity in relation to frequency domain. The second experiment combines a High Intensity Focused Ultrasound (HIFU) to generate SAW and optical coherence tomography (OCT) for detection of SAW. 2% and 5% agar phantom were tested.
Results of these two sets of experiments show that SAW method can be used to assess elasticity of soft tissues. Processing SAW results give phase velocity that indicates the elastic properties of material under study.
TABLE OF CONTENTS
Click to expand Table of Contents
CHAPTER 1: INTRODUCTION
1.1 INTRODUCTION
1.2 AIM OF PROJECT
1.3 THESIS STRUCTURE
CHAPTER 2: LITERATURE REVIEW AND BACKGROUND
2.1 INTRODUCTON
2.2 BACKGROUND: HUMAN SKIN
2.3 ELASTICITY OF THE SKIN
2.4 SKIN ANATOMY: SOFT TISSUE
2.4.1 Structure of Epidermis
2.4.2 Epidermis Elasticity Features
2.4.3 Structure of Dermis
2.4.4 Dermis Elasticity Features
2.4.5 Structure of Hypodermis
2.4.6 Hypodermis Elasticity Features
2.5 SKIN DISEASE
2.5.1 Melanoma
2.5.2 Psoriasis
2.6 MECHANICAL PROPERTIEES OF SOFT TISSUE
2.7 TISSUE ELASTICITY MEASUREMENT
2.8 MEDICAL IMAGING AND METHODS
2.8.1 High Intensity Focused Ultrasound (HIFU)
2.8.2 Optical coherence Tomography (OCT) imagining method
2.8.3 Optical Coherence Elastography (OCE) imagining method
2.9 ANSYS AND FINITE ELEMENT METHOD
2.10 CONCLUSION
CHAPTER 3: MECHANICAL WAVES
3.1 INTRODUCTION
3.2 TYPES OF WAVES
3.2.1 Longitudinal waves
3.2.2 Shear waves
3.2.3 Surface acoustic waves
3.3 CHARACTERISATIONS AND APPLICATIONS OF SAW
3.3.1 SAW Filters
3.3.2 SAW Resonators
3.4 GENERATION AND DETECTION OF SURFACE ACOUSTIC WAVES
3.4.1 Contact Technique
3.4.2 Non-contact Technique
3.5 CONCLUSION
CHAPTER 4: METHOD AND PROCEDURE
METHODOLOGY OF EXPERIMENT (A)
4.1 INTRODUCTION
4.2 BRIF INTRODUCTION OF ANSYS
4.3 FINITE ELEMENT ANALYSIS USING ANSYS
4.3.1 Pre-processing
4.3.2 Solution
4.3.3 Post processing
4.4 METHOD AND PROCEDURE
4.4.1 ANSYS Setup
4.4.2 Modelling and Geometry
4.4.3 Mesh
4.4.4 Solutions
4.5 ANSYS SOULTION AND FINITE ELEMENTE RESULTS
METHODOLOGY OF EXPERIMENT (B)
4.6 MAKING AGAR PHANTOM
4.6.1 Preparation of 2% and 5 % agar
4.6.2 Setup the experiment
4.7 CONCLUSION
CHAPTER 5: SAW SIGNAL PROCESSING AND DISCUSSION OF RESULTS
5.1 SAW SIGNAL PROCESSING
5.2 PROCESSING THE SAW USING THE SPECTRAL ANALYSIS
5.3 INVERSION PROCESS
5.4 RESULTS AND DISCUSSION
5.5 EXPERIMENT (A)
5.5.1 Model (A)
5.1.2 Model (B)
5.1.3 Model (C)
5.6 EXPERIMENT (B)
5.6.1 2% Agar
5.6.2 5% Agar
5.7 COMPARION OF THE PHASE VELOCITY
5.8 CONCLSION
APPENDIX A
APPENDIX B
APPENDIX C
REFERENCES
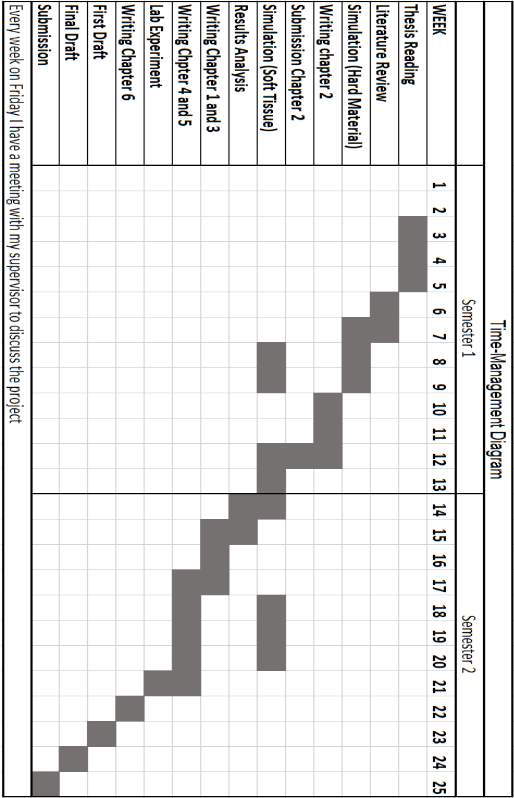
TIME MANAGEMENT DIAGRAM
RISK ASSESSMENT
LIST OF FIGURES
Figure 2. 1 Human skin and the appendages (Rook and Burns, 2010)
Figure 2. 2 Schematic organization of ECM components (Breitkreutz et al., 2013)
Figure 2. 3 Anatomy of skin (source WebMD 2014)
Figure 2. 4 Epidermis keratinocyte layers (Rook and Burns, 2010).
Figure 2. 5 Melanoma
Figure 2. 6 the linear stress-strain curve in skin with the related collage fibres morphology (source Holzapfel 2000:4)
Figure 2. 7: Methods of testing to estimate Young’s Modulus in skin (Kalra, Lowe and Aljumaily 2016).
Figure 2. 8 Principle of HIGH for tumour treatment
Figure 2. 9 Physical principles of HIFU (Chaussy and Thüroff 2011)
Figure 2. 10 A schematic of the transducer (Moy 2008)
Figure 2. 11 An OCT system based on a Michelson interferometer (Tomlins and Wang 2005)
Figure 2. 12 The position of OCE among various non-invasive imaging techniques in terms of scale of detection.
Figure 3 1 Particles movement in SAW
Figure 3 2 Simple sound Waves
Figure 3 3 Basic SAW device
Figure 3 4 Bandpass Filter Characteristic
Figure 4. 1 ANSYS setup 1
Figure 4. 2 ANSYS setup 2
Figure 4. 3 PLANE 55 (a) and 42 (b) in ANSYS (L’Etang & Huang Z, 2006)
Figure 4. 4 Materials setup in ANSYS
Figure 4. 5 Sample 1 in Model A (Fat only)
Figure 4. 6 Sample 2 in Model B (Epidermis + Fat)
Figure 4. 7 Sample 3 in Model C (Fat + Dermis)
Figure 4. 8 Model D (Epidermis +Dermis+ Fat)
Figure 4. 9 Model E (Epidermis and dermis separate + fat)
Figure 4. 10 Mesh of sample with three different layers
Figure 4. 11 ANSYS setup for the solution
Figure 4. 12 ANSYS Thermal results
Figure 4. 13 structural analysis results
Figure 4. 14 Detection points used in each 20mm in Y-direction
Figure 4. 15 SAW the original signal data from ANSYS
Figure 4. 16 Importing data from ANSYS to Excel
Figure 4. 17 Displacement of SAW in single layer model
Figure 4. 18 Displacement of SAW in double soft layers
Figure 4. 19 Agar mixture preparation 1
Figure 4. 20 Agar Mixture preparation 2
Figure 4. 21 HIFU Transducer device
Figure 4. 22 Experiment setup
Figure 5. 1 Phase velocity in frequency domain for fat
Figure 5. 2 Phase velocity of SAW in frequency domain for Epidermis and fat
Figure 5. 3 Phase velocity in the frequency domain for dermis and fat
Figure 5. 4 Phase velocity for 2% agar
Figure 5. 5 Phase velocity for 5% agar
LIST OF TABLES
Table 2. 1 Mechanical properties and Biochemical data of some connective tissue (Holzapfel 2000:3)
Table 2. 2 Young’s Modulus for Various of human tissue
Table 4. 3 Mechanical properties for the double layers modal
Table 4. 4 Properties of tissue mimicking agar phantoms used in FE
CHAPTER 1: INTRODUCTION
1.1 INTRODUCTION
Changes in mechanical properties of human tissues are often observed in some diseases. The ability to measure these mechanical properties can be useful in the diagnose and treatment of, for example, cancer and scleroderma. Accurate diagnostic tools and methods can improve treatment for deadly skin conditions can result in high cure rate up to 90%-100% (Li 2013).
A number of elastography methodologies and technologies are developed recently to obtain qualitative and quantitative assessments of mechanical properties. Surface acoustic wave (SAW) method has been used to assess quantitatively Young’s Modulus, in which an ultrasound transducer is used to detect SAW. Phase velocity values are related to Young’s Modulus which can be used to obtain elasticity information of the skin layers.
1.2 AIM OF PROJECT
The aim of this project is to use Finite element analysis to simulate SAW in different tissues, single and double, with different properties in the first set of experiments. The project will make use of ANSYS mechanical ADPL to simulate the thermal and structural of the multi layers of soft tissue. Then, the software Matlab is used to analysis the data found in the ANSYS, and plot the graphs of waveform and phase velocity of the Surface Acoustic Wave (SAW). The second experiment will test 2% and 5% agar phantoms with use of HIFU to generate SAW waves and an OCT to detect them. The results are then processed to obtain the phase velocity graphs of SAW.
1.3 THESIS STRUCTURE
Chapter 2 of the thesis introduces the literature review, including background and structure properties of human skin, elasticity of the skin and skin disease, mechanical properties of soft tissue, elasticity measurement of the tissue, and medical imaging methods and techniques, including High Intensity Focused Ultrasound (HIFU), Optical Coherence Tomography (OCT) imagining method, and Optical Coherence Electrography (OCE) imagining method.
Chapter 3 introduces mechanical weaves: longitudinal, shear and surface waves. It also discusses the characteristics and applications of SAW in particular, and its generation and detections.
Chapter 4 has two main sections discussing the two experiments A and B. Methodology of experiment (A) includes introduction and background of ANSYS and details of the finite element analysis, element analysis (FEA) set up, simulation procedure, and results. Methodology of experiment (B) includes preparation of the phantoms, set of the experiment, and the procedure of the experiments.
Chapter 5 present the signal processing of SAW. It discusses SAW signal processing, Spectral Analysis of SAW, and de-noising techniques. It then discusses the results obtained for experiment A and B that were discussed earlier in chapter 4.
CHAPTER 2: LITERATURE REVIEW AND BACKGROUND
2.1 INTRODUCTON
This chapter introduces elasticity in human skin and the mechanical properties of soft tissue. It investigates the biological components of soft tissue that influences the elasticity in section 2.3. Section 2.4 introduces the measurements used in determining elasticity. Several medical imaging methods is introduced in section 2.5.
2.2 BACKGROUND: HUMAN SKIN
Human skin is divided in different parts to conduct this study. This study mainly concentrates on the soft tissue in the human skin. Skin is the biggest organ. Knowledge on characteristics of the human skin can that help in understanding the pathophysiology, which is vital for diagnosis and treatment of conditions such as skin cancer and scleroderma (Gennisson et al., 2004; Zhang, Kinnick, and Greenleaf, 2008). Many skin condition are diagnosed qualitatively through palpation or visual examination by an experienced dermatologist. Kirkpatrick et al (2006) notes that the level of strain response varies with the kind of pathology. The basic skin features and anatomy are examined in the following sections based on available literature.
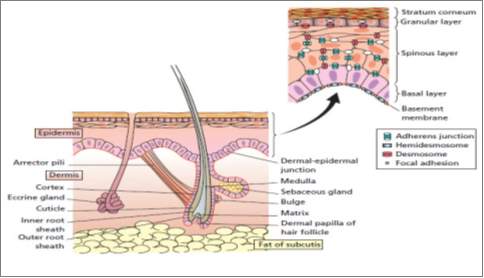
Figure 2. 1 Human skin and the appendages (Rook and Burns, 2010)
Skin constitutes the body’s largest organ of the human adult body. It comprises 16% of adult weight, in which it supports internal organs and protects our body (Holzapfel 2000, 3). Skin is one of the soft tissues, i.e. tendons, ligaments, blood vessels and articular cartilages, which are distinct from hard tissue, e.g. bones, in their flexibility and soft mechanical properties. Skin is a protective barrier for internal organ from outside bacteria, toxins, ultraviolet radiation. It also has other functions including; sensory perception, immunologic surveillance, thermoregulation, and fluid loss control (Amirlak 2015, 1). Skin thickness ranges from 0.05mm (in post auricular region) to 1.5mm in the palms and feet, and differ from male to female. Skin thickness also differ according to age with children having thin skin (Amirlak 2015, 1).
Desmosomes are the adhesion complexes between the cells bridging them by linking intermediate filaments of adjacent cells (Figure 1). In the epidermis, the desmosomes are the major adhesion complexes which tightly bind keratinocytes through keratin intermediate filaments and maintain the structure of epidermis (Holthofer et al., 2007). The major components of desmosomes are desmosomes cadherin, armadillo proteins and the plakins (Green and Simpson, 2007). Other than providing cell adhesion and integrity to the tissue, desmosomes are reported to have a key role in intracellular signal transduction, influencing the cell proliferation and differentiation (Garrod and Chidgey, 2008).
2.3 ELASTICITY OF THE SKIN
Skin elasticity is defined as the capability of the skin to stretch, without breaking, then revert to its usual state or firmness. Elastosis, which is a reduction in skin elasticity, comes with age (Menon, 2015). Because the soft tissue are created from different materials, such as collagen and elastin, which makes them to have properties that are coordinate and direction dependent, which is described through viscoelasticity. Delalleau et al (2007) stated that the main element of dermis are the collagen fibbers that gets oriented to the direction of stress when exposed to small strain. However, the epidermis is a thin coating made of stratified epithelium. The skin has fibers that are systematized in several biaxial layers of the thin membranes. The most critical mechanical features of the skin resist friction. The mechanical attributes change with age, disease, hydration, exposures and orientation and site. The changes that happen in the human skin produce deformation is twofold: first, the fibers rotate and stretch, and second, under pressure the matrix fluid is forced out between fibers.
The following are the mechanical properties of the dermal section of the skin.
- Collagen fibers make up the largest part of the dermis (about 77% of the dry weight – fat-free) and creates an irregular web of coiled fibers which run parallel to skin surface. The collagen fibers run separate in most areas but are held together by ground substance. Collagen has low extensibility, high strength (1.5-3.5 Mpa tensile strength) and high stiffness.
- The elastin fibers are less stiff compared to the collagen and have reversible strains for over 100%.
- Reticulin appears in smaller amount, but it has similar morphology and molecular structure as collagen and thus has similar properties.
- Ground substance accounts for the dermis’ viscoelastic behavior, contributing to its tensile strength (Menon, 2015).
The Fibroblasts are mainly linked to the macromolecules ad are located in the ECM network (Delalleau et al., 2007). The fibroblast is named bioactive cells form active cells while they are named fibrocystic if they differentiate to create active cells. Fibrocystic work to bar the creation of ECM like collagens, glycosaminoglycans, and hyaluronic acids, while fibroblasts take part in the regenerative processes through active syntheses of ECM.
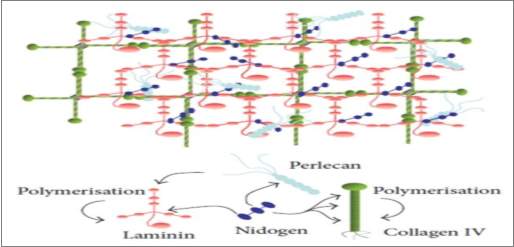
Figure 2. 2 Schematic organization of ECM components (Breitkreutz et al., 2013)
Fibronectin, collagens, and laminins have a critical role since they control the composition of the basal lamina and affect the dermal-epidermal interactions (Delalleau et al., 2007). The dermal fibroblasts take part on the epidermalization through the production of a diffusible factor that controls the epidermal survival and proliferation.
2.4 SKIN ANATOMY: SOFT TISSUE
Skin has three layers: Epidermis, Dermis, and Subcutaneous layer as shown in (Figure 1). The thickness of the epidermis varies between 0.5 mm in the eye lids to 1.5 mm in the sole of the foot while the thickness of the dermis is 1.5 to 4 mm [SEER Training]. The hypodermis or subcutaneous layer varies across the body and from person to person (Li 2013: 11).
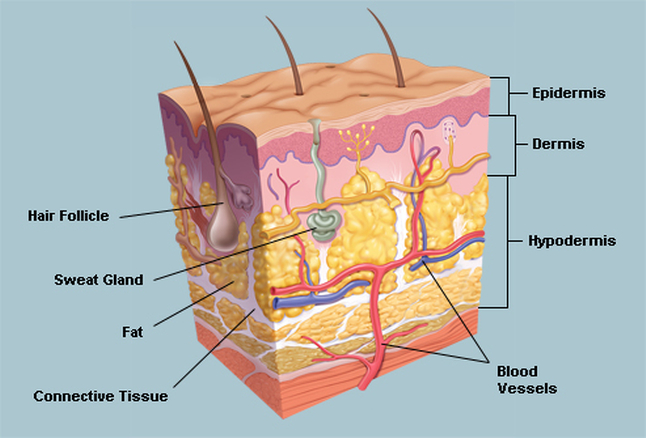
Figure 2. 3 Anatomy of skin (source WebMD 2014)
2.4.1 Structure of Epidermis
The epidermis is the outer layer that gives skin its tone. It provides protection against from infections, dehydration and trauma (Li 2013, 10).
The epidermis is made up of densely arranged squamous epithelium, which are just 70-140 nm thick. Epidermis is made up of the keratinocyte as its main cell type. The keratinocyte growly closely to each other and leave only little space for the ECM, to create a compact barrier against the surrounding (Yoshida et al., 2015, p. 92). Also, epidermis is a periodically self-renewing element. Keratinocytes repeatedly proliferate and differentiate outwards on the external surface changing from the polygonal viable cells to the non-viable flattened squames filled with keratin. The process of differentiation is referred to as keratinization (Menon, 2015). The human epidermis is sectioned into five layers (Yoshida et al., 2015).
Stratum basal is composed of a single layer created from cuboidal keratinocytes at the lowest of epidermis, and fixed to the dermis through basement membrane. The layer’s keratinocytes hold the largest proliferation potential, and significant contribute to the high regenerative efficiency seen in the epidermis (Rook and Burns, 2010). During the cell division, two daughter cells are produced where one of them replaces the original basal cell and is anchored on the basement membrane, and the other moves upwards through other layers and is ultimately sloughed off. The neighboring keratinocytes are interlocked tightly through the ton fibrils linked ay the desmosomes that are hugely distributed over skin plasma membranes. Finally, the Basal cells are attached to basement membranes through hemi desmosomes.
The stratum spinosum is made up of polyhedral keratinocytes layers having many cytoplasmic “prickles” (Gennisson et al., 2004, p. 892). Also, the cell are packed tightly close to each other via the desmosomes located at the prickles, offering the required coherence and tensile strength in epidermis. Cytokeratin is continually produced and it aggregates to create the ton fibrils that merge on the desmosomes (Yoshida et al., 2015, p. 95). The stratum granulosum based Keratinocytes experience significant stricture changes. Organelles and nuclei like Golgi apparatus, ribosomes, and mitochondria start to degenerate. Further, the Keratin filament collects into more associate and compact with the dense keratohyalin granules at the cytoplasm (Rook and Burns, 2010). The death of the cells started near the top of the stratum granulosum, where the lysosomal enzymes that takes part in the keratinization procedure are freed.
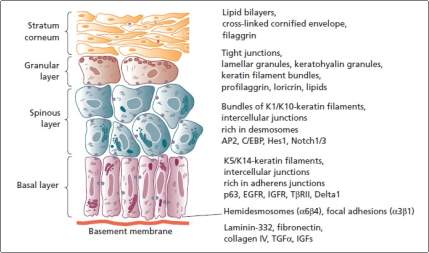
Figure 2. 4 Epidermis keratinocyte layers (Rook and Burns, 2010).
The stratum lucidum is a transition layer between the outer most stratum corneum and the stratum granulosum. It is a stratum granulosum that can only be seen in the thick, hairless skin, where cell boundaries are carefully closely observed. Finally, the stratum corneum is described as a final product of the process of keratinization (Yoshida et al., 2015, p. 98). Stratum corneum is formed from compressed, cornfield layers from dead keratinocytes that lack nuclei and other organelles but is jam-packed with keratin. The cornfield cells, referred as coenocytes, are interlocked by grooves, bridges and microvilli. The innermost layers of the stratum corneum keep the desmosome junctions, creating a waterproof physical barrier for the skin.
2.4.2 Epidermis Elasticity Features
Epidermis is fixed to dermis via basement membrane that is composed of the plasma membrane of basal lamina, stratum basal, and lamina fibroreticularis that merge with the dermis. Further, the basal lamina is divided into the electron-lucent lamina lucida layer (40-50nm) and the electron-opaque lamina densa layer (70nm). The Intracellular cytokeratin filaments link the basal keratinocytes to basement membrane through inserting into hemi desmosomes that connect through anchoring filaments into the lamina densa and across the lamina lucida. The anchoring fibrils created from collagen VII extend out of the lamina densa to a lamina fibroreticularis, and interlaock to collagen I and III at the papillary dermis (Menon, 2015). The junction between epidermis and dermis is described by a downward fold of the epidermis that interdigitates through the upward projection of dermal papillae, creating an undulating interface named the rete ridges. The arrangement eases adhesion between the dermis and epidermis through an increase in are of contact, and is thus prominent is skin that is given higher shear stress like at the soles of the feet.
2.4.3 Structure of Dermis
The second layer is the dermis. This layer contains different structures, unlike the first layer, including hair follicles, blood vessels, glands and nerves. The connective tissue has capillaries, elastic fibres, reticular fibres, and collagen. The dermis sustains the epidermis and regulate temperature (Li 2013, 11 and Amirlak 2015, 1).
The dermis is a soft irregular connective tissue that has matric created from a network of elastic fibers and collagen, implanted in the amorphous ground substance of the glycoproteins, glycosaminoglycan, and water (Delalleau et al., 2007).
The dermis works like the strong support for the epidermis and offers the skin its distinctive viscos-elastic mechanical features. The main cell category in the dermis is the fibroblasts and exist at low cell densities. The Fibroblasts produce the different glycosaminoglycan (GAGs) and ECM proteins that are in then dermis. However, the fibroblasts do not differentiate, unless stimulated via wound healing, like the keratinocytes. Also, the dermis has mast cells, white blood cells, and macrophages.
2.4.4 Dermis Elasticity Features
The dermis gives the largest part of the mechanical features due to the large number and arrangement of elastin fibers and collagen in it. Further, the dermis supports tissues for blood vessels, nerves and skin appendages and hair follicle units. It is divided into reticular dermis and papillary dermis (Imokawa, and Ishida, 2015, p. 7753). The papillary dermis is considerably thin than the reticular dermis and stacked below the basement membrane and is created of finely meshed collagen, mainly type III, and elastin bundles. It holds an extensive capillary network that considers papillary folds, offering the dermis the nutrients and allows waste removal. The reticular dermis composes the greatest part of the dermis and has irregular, thick collagen bundles created from parallel fibers, mainly of type I that are aligned in a plane that is parallel to the skin’s surface.
2.4.5 Structure of Hypodermis
The third layer is called hypodermis or subcutaneous layer, though it can be argued that there is no clear division between dermis and hypodermis. The hypodermis is located below the dermis and is made up of vascularized adipose tissue which offer insulation and protects the underlying tissues from any external impacts. Hypodermis is a subcutaneous fibrofatty that is loosely linked to the dermis (Rook and Burns, 2010). However, its thickness varies depending on sex, age, and anatomical site, nutritional and endocrine status of the person. The hypodermis functions like the protective cushion and insulating layer and makes up about 10% of an individual’s body weight. It contains well-vascularized, loose, areolar connective tissue and adipose tissue. It functions as a fat storage and provides insulation and cushioning for the integument (Betts et al 2016). Li (2013: 11) explains that this layer, mechanically, allows the overlaying skin to move both vertically and horizontally, and allow for the attenuation and dispersion of externally applied pressure.
2.4.6 Hypodermis Elasticity Features
Tensile loading happens if the skin is drawn in a plane of the surface, such as the closing of a wound using suture provokes tension crosswise. Also, it may be caused by various loading like shearing and swelling (Nicolle, Decorps, Fromy, and Palierne, 2016). For instance, in the distal region of the residual limb or near adherent scar tissue.
2.5 SKIN DISEASE
The skin, like other organs, of the body, is subject to diseases which are idiopathic or symptomatic. The affections which are symptomatic are dependent upon general pathological conditions, the so-called blood diseases, or upon the diseases of particular organs. Skin disorders can be a result of an infection, a cancer or an allergy (Li 2013:11). Skin diseases can either be primary in skin, e.g. psoriasis, or can be secondarily to an internal disease, e.g. the malar rash accompany lupus erythematosus, and varies in severity (McConnell 2007: 651). What is really relevant to our dissertation is that different diseases can have an effect on the skin properties by which the pathological changes can result in changes in the elastic properties of the skin (the stiffness of the skin or the thickness of the skin layers) (Li 2013: 11). Thus, measuring the elasticity of the skin can help in diseases diagnosis or treatment as Li et al (2012: 831) argue that cancer, for example, can be detected by changes in stiffness of tumour compared with the surrounding area.
2.5.1 Melanoma
Genetic testing has reported that many cases of melanoma, both sporadic and familial, are instigated by mutation of a tumor suppressor gene named p16. The tumor suppressor gene works to control cell growth and proliferation to allow the cells to grow in a scheduled and controlled manner (Leiter, Eigentler, and Garbe, 2014, pp. 130). After the gene like p16 is mutated, the cells are unable to divide in a controlled way and thus creates a tumor (Menon, 2015). Melanoma, like any other cancer, has stages 0 to stage IV. The abnormal melanocytes are seen in the epidermis layer forming a tumor that spread to the nearby tissues skin.
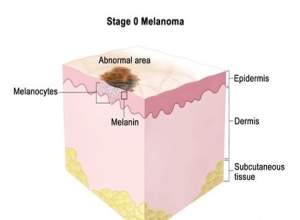
Figure 2. 5 Melanoma
Melanoma’s stage is separated to stage IA and stage IB. Stage IA forms as a small tumour, less than a millimetre while stage IB could have an ulceration and be 1-2 millimetres thick. Stage II has two separations stage IIA, stage IIB, and stage IIC (Leiter, Eigentler, and Garbe, 2014, pp. 133). Melanoma in stage III might have any thickness and might have an ulceration, which spread to various lymph nodes. Melanoma at stage IV has spread very wide, moving particularly to the soft tissue, liver. Lung and brain. Further, it could spread to different parts of the skin that are located far from the first location of the tumour.
2.5.2 Psoriasis
Psoriasis is described as an inflammatory dermatosis shown by erythematous, greatly demarcated plaques and papules that cover silvery white scales that affect the scalp and skin at back of knees and elbows. According to Pouplard et al. (2013), Psoriasis is a common immune-mediated illness (Osmancevic et al., 2014). Environmental and genetic influences participate to the development of the condition. Some of the environmental factors include alcohol consumption, streptococcal tonsillitis, and trauma (Pouplard et al., 2013). Psoriasis is known to alter the life cycle of the skin cells. It leads to a fast build up at the skin’s surface.
2.6 MECHANICAL PROPERTIEES OF SOFT TISSUE
Mechanical properties of soft tissue, including skin, are influenced by the constituents of the tissue, and these constituents are collagen, elastin, the hydrated matrix of [1]proteoglycans and the topographical site and function of the tissue (Holzapfel 2000, 2). Collagen and elastin are two proteins that play role in the elasticity of skin as Table 1 below we can see mechanical properties of some types of connective tissue and its biochemical data:
Table 2. 1 Mechanical properties and Biochemical data of some connective tissue (Holzapfel 2000:3)
| Material | Ultimate tensile strength (MPa) | Ultimate tensile strain (%) | Collagen (% dry weight) | Elastin (%dry weight ) |
| Tendon | 50-100 | 10-15 | 75-85 | <3 |
| Ligament | 50-100 | 10-15 | 70-8 | 10-15 |
| Aorta | 0.3-0.81 | 50-100 | 25-35 | 40-50 |
| Skin | 1-20 | 30-70 | 60-80 | 5-10 |
| Articular cartilage | 9-40 | 60-100 | 40-70 | – |
Holzapfel (2000:4) explains that the tensile response is nonlinear stiffness and strain rate determines tensile strength whilst soft tissue at the same time can undergo deformation. The mechanical properties of soft tissues are elasticity, Poisson ratio and density (Li 2013: 14),[2] for which only elasticity is variable and indicative of stiffness of skin in different pathological conditions.
2.7 TISSUE ELASTICITY MEASUREMENT
Elasticity is measured by Young’s modulus (elasticity modulus) in which it describes the longitudinal deformation of a material by calculating strain caused by stress (Tianjin & Feng 2008).
Stress is a force per unit area applied to material, which is defined as:
- Number of steps= 2000
- Frequency= write every sub step
- Define load in uniform Temperature =300
- Finally selected everything and solve current LS.
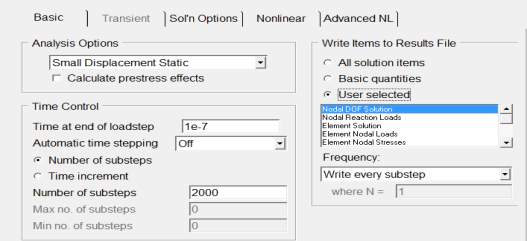
Figure 4. 11 ANSYS setup for the solution
4.5 ANSYS SOULTION AND FINITE ELEMENTE RESULTS
In the figures below shows the steps of the Finite Element Analysis solutions to obtain the required results.
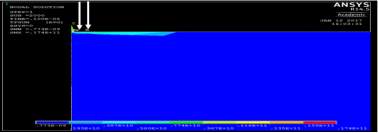
Figure 4. 12 ANSYS Thermal results
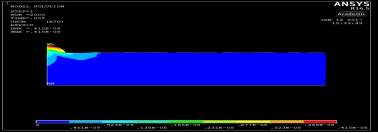
Figure 4. 13 structural analysis results

Figure 4. 14 Detection points used in each 20mm in Y-direction
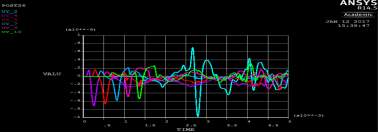
Figure 4. 15 SAW the original signal data from ANSYS

Figure 4. 16 Importing data from ANSYS to Excel
The results of the experiments are as follow:
1. Single layer model
In figure below shows the displacement in Y- direction of the signal 2% of agar layer at different detection points across the timeline. In total, there are 6 detecting points for the first experiment single layer. The detecting points have been set in every 20 mm between the points, and 5mm from the laser pulse source. The displacement in the figure shows the propagating of surface acoustic waves in a 2 % of agar and it shows the wavelength in soft tissue in single layer is faster than propagating in double layers.
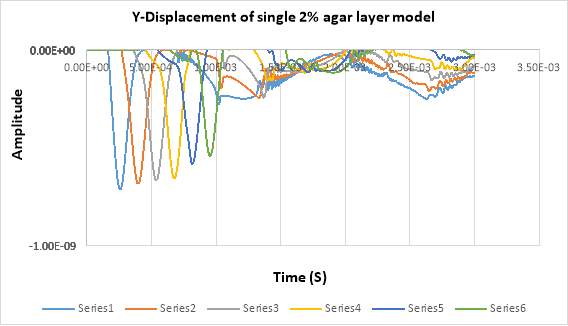
Figure 4. 17Displacement of SAW in single layer model
2. Two layers model
In this experiment used (Epidermis + dermis with fat) and one layer thickness used 0.0019m. In the figure below shows the displacement in Y- direction of the double layer using 2% of agar at different detection points across by the time. There are 4 detecting points in total for the second experiment. The detecting points have been set in every 10 mm between the points, and 5mm from the laser pulse source. The displacement in the figure shows the propagating of surface acoustic waves in 2 % of agar.
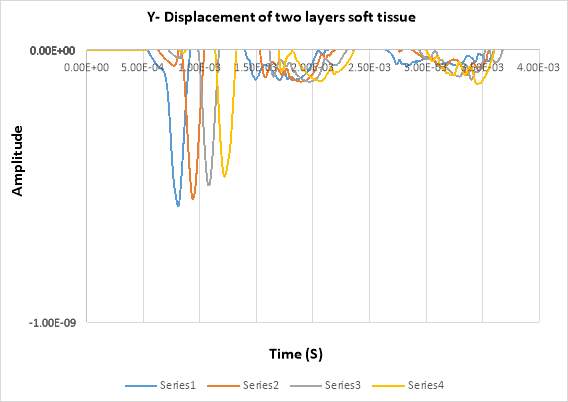
Figure 4. 18 Displacement of SAW in double soft layers
By comparing with single model (fat), it can be seen that the amplitude in two layers has minimum values of (-5.5e-10) whereas, in the single model has (-7.0e-10).
By analysing the wave length it can be seen that the wave lengths of single layer has deeper than the wave length of two layer model.
METHODOLOGY OF EXPERIMENT (B)
4.6 MAKING AGAR PHANTOM
As mentioned in section A the mechanical properties of soft tissue is important to understand the elasticity in human skin. For example, most of skin pathological changes due to the changes of thickness and elastic properties of the skin.
In this part of project, surface acoustic wave elastography is combined with using high intensity focused ultrasound in low energy laser pulse to investigate elasticity of the skin.
In Tissue mimicking phantoms are specifically designed material that can closely mimic certain physical properties of the human tissues. In order to examine how tissue is affected by medical imaging procedures such as high intensity focused ultrasound (HIFU), tissue-mimicking gelatine is materials such as agar are often used as a substitute for living tissue, and can be manufactured in the lab to have similar mechanical properties of human skin as you can see in the figure below.
Table 4. 4 Properties of tissue mimicking agar phantoms used in FE
| Parameters of Material | 2% agar phantom | 5% agar phantom |
| Young’s Modulus | 18000 | 134200 |
| Poisson’s Ratio | 0.47 | 0.47 |
| Density | 1040 | 1040 |
| Thermal Expansion | 3.0e-4 | 3.0e-4 |
| Thermal Conductivity | 3590 | 3590 |
| Specific Heat | 24 | 24 |
4.6.1 Preparation of 2% and 5 % agar
Before starting the experiment the 2% of agar phantom has been made in the lab.
There are a number of steps to prepare the agar phantom:
The agar phantom is made in the lab by mixed with 2% of agar powder and little bit titanium and 100 ml of water. The liquid is mixed by a special device for about 20 minutes the reason is to remove all the bubbles and get a good result.

Figure 4. 19 Agar mixture preparation 1
After the agar mixed probably, the liquid has been pour in small circle plastic about 5 cm width.

Figure 4. 20 Agar Mixture preparation 2
The agar as well has been shaped in the base agar phantom at miniature HIFU Transducer device. The thickness of the base is about 5cm.

Figure 4. 21 HIFU Transducer device
4.6.2 Setup the experiment
Before discussing the experiment, the setup of the experiment observed the following:
- High intensity focused ultrasound (HIFU) is the main energy used to generate SAW waves inside the sample.
- We used it to track SAW inside the sample. The most important point is to obtain accurate structure images.
- We used low voltages or low energy.
The Phs-OCT has been used in this experiment, which was develop in bioinstrumentation group at University of Dundee. The OCT is used for detection of the SAWs waves.
The experiment is divided into two main parts, the first one surface acoustic waves generations and the second part is detection of the waves. The sample of 2% agar has been set up above the miniature HIFU transducer.

Figure 4. 22 Experiment setup
This experiment as shown in figure 4.22 above used HIFU, in low voltages (low energy), to generate vibrations to a localised point. The vibrations then generate waves (SAW). The OCT method then detect and are used to calculate the speed of the surface acoustic waves within the sample which is related to elasticity. The results of this experiment is discussed with experiment A in chapter
4.7 CONCLUSION
We discussed in this chapter the methodology and procedure for each of experiments A and B. Mistakes and errors in the data in the conduction the experiments can lead some inaccurate results that may be ignored. Combining to sets of experiments, one in ANSYS and one in Lab in a 2% and 5% agar phantoms increases the accuracy of our results.
CHAPTER 5: SAW SIGNAL PROCESSING AND DISCUSSION OF RESULTS
5.1 SAW SIGNAL PROCESSING
We discuss the processing of SAW signals to calculate phase velocity from raw data. Lower frequency surface acoustic waves can propagate with lower phase velocity as well. However, the higher frequency surface acoustic wave propagates at the surface layer with a higher phase velocity. This section introduces the principles and methods used in processing surface acoustic wave signals to investigate the mechanical properties of each layer to reach the aim of estimating the phase velocity.
5.2 PROCESSING THE SAW USING THE SPECTRAL ANALYSIS
Processing full waveform data obtained from the experiments above in chapter 4, we need two operations: frequency bandwidth and phase velocity as frequency.
The phase velocity in frequency can be determine by detecting the wave traveling distance and the travel time. The frequency bandwidth is determined by spectral analysis. After results are obtained from ANSYS, it is necessary to process the raw data to get the phase velocity and frequency bandwidth. Several processing methods have been applied to determine the phase velocity of the surface acoustic waves. The spectral analysis is used in this experiment to process SAW which is known as SASW to process phase velocity and frequency bandwidth. The nature of SAW simulation and detection usually results in noise being detected. There are different de-noising methods are used along SAW signal processing. The result of the signals processing obtain a phase velocity curve that reflects the elasticity and geometry information of the sample (Li 2013, 81-2).
5.2.1 Spectral Analysis of SAW (SASW)
Transient signal is decomposed in its components in spectral analysis which obtain a broadband dispersion curve. It is done in a number of locations with equal spacing, and the difference between any two points can be used to calculate a phase velocity data of the material. The final phase velocity curve is obtained by averaging. The frequency range is determined based on the frequency content in the power spectrum with the cut-off frequency of 2% agar phantom SAW signal chosen at 10 kHz (Li 2013: 83).
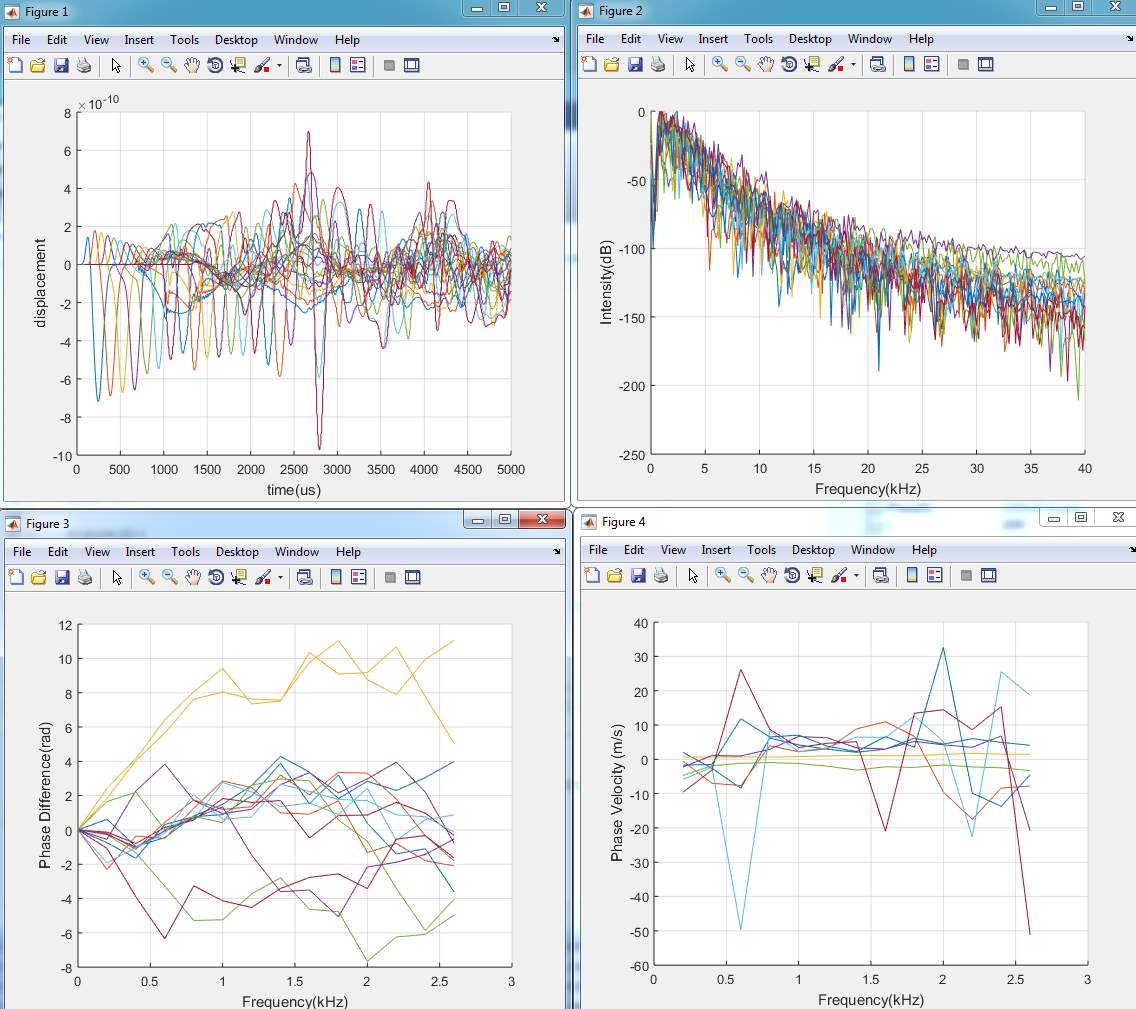
The phase velocity is calculated. There are two SAW signals
%plot the Phase Difference
Figure (3)
figure(3);
hold on
for i=1:c-2
plot(Freq(1:14)/1e3,unPD1(1:14,i));
%plot(Freq(1:21)/1e3,unPD2(1:21,i));
end
xlabel(‘Frequency(kHz)’);
ylabel(‘Phase Difference(rad)’);
grid on
hold off
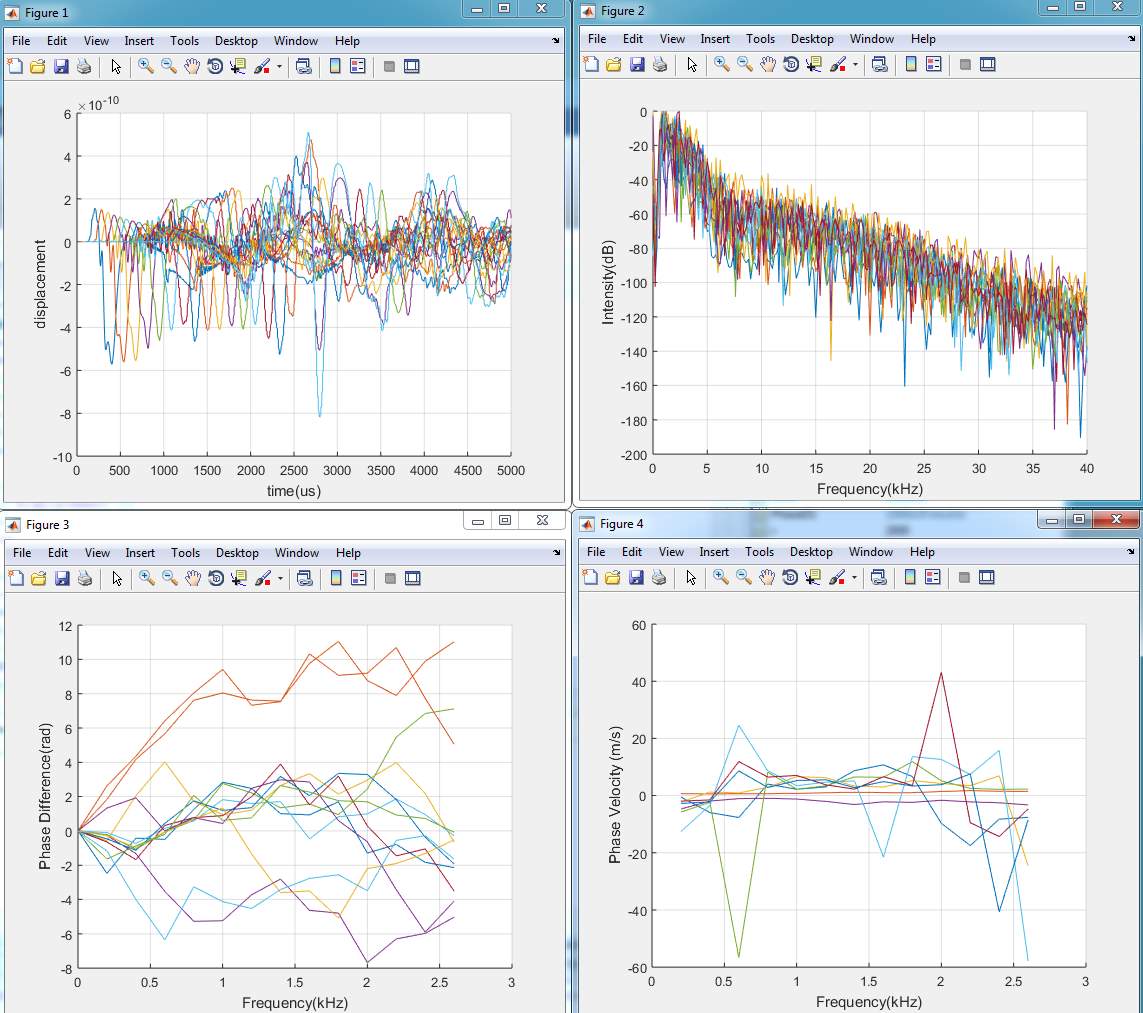
%Phase velocity curve of each frequency
Figure (4)
figure(4);
hold on
for i=1:8
Cr1(:,i)=Distance*2*3.14*Freq’./unPD1(:,i);
%Cr2(:,i)=Distance*2*3.14*Freq’./unPD2(:,i);
plot(Freq(1:14)/1e3,Cr1(1:14,i)/1000);
%plot(Freq(1:21)/1e3,Cr2(1:21,i)/1000);
end
xlabel(‘Frequency(kHz)’);
ylabel(‘Phase Velocity (m/s)’);
grid on
hold off
AveargePV=mean(Cr1(3:14,:)); %average phase velocity unit mm/s
disp(AveargePV);
APPENDIX C
1. Model (A)
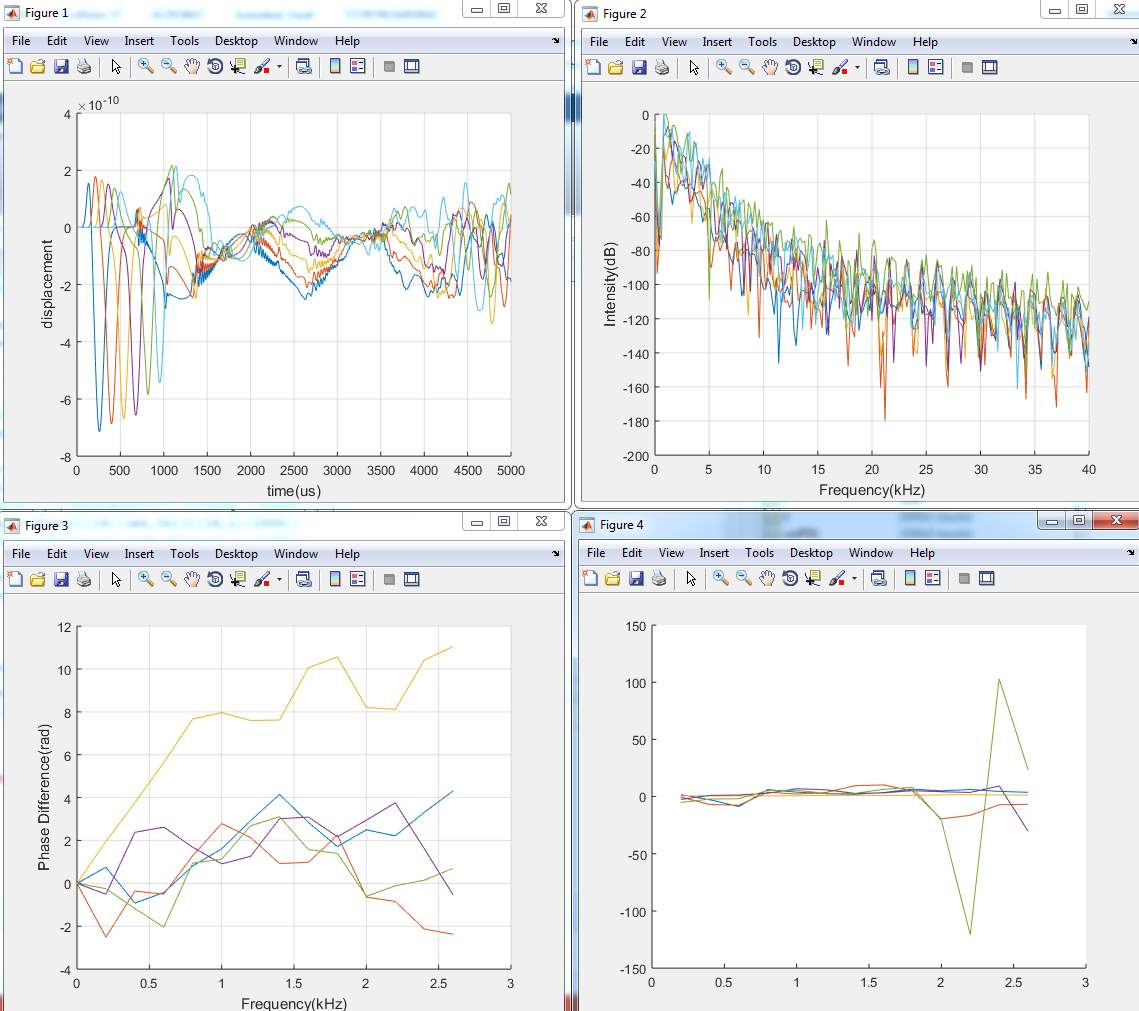
2. Model (B)
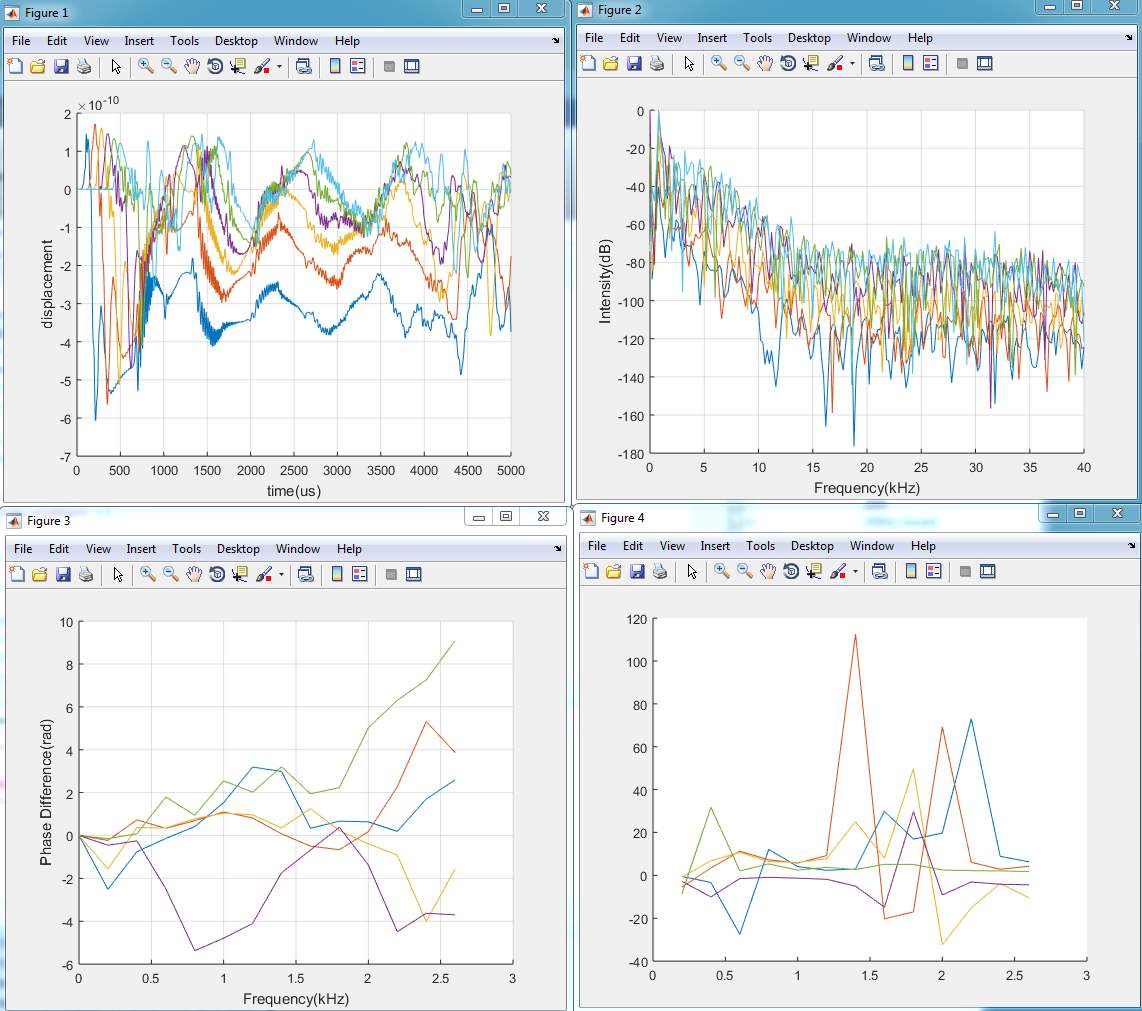
3. Model (C)
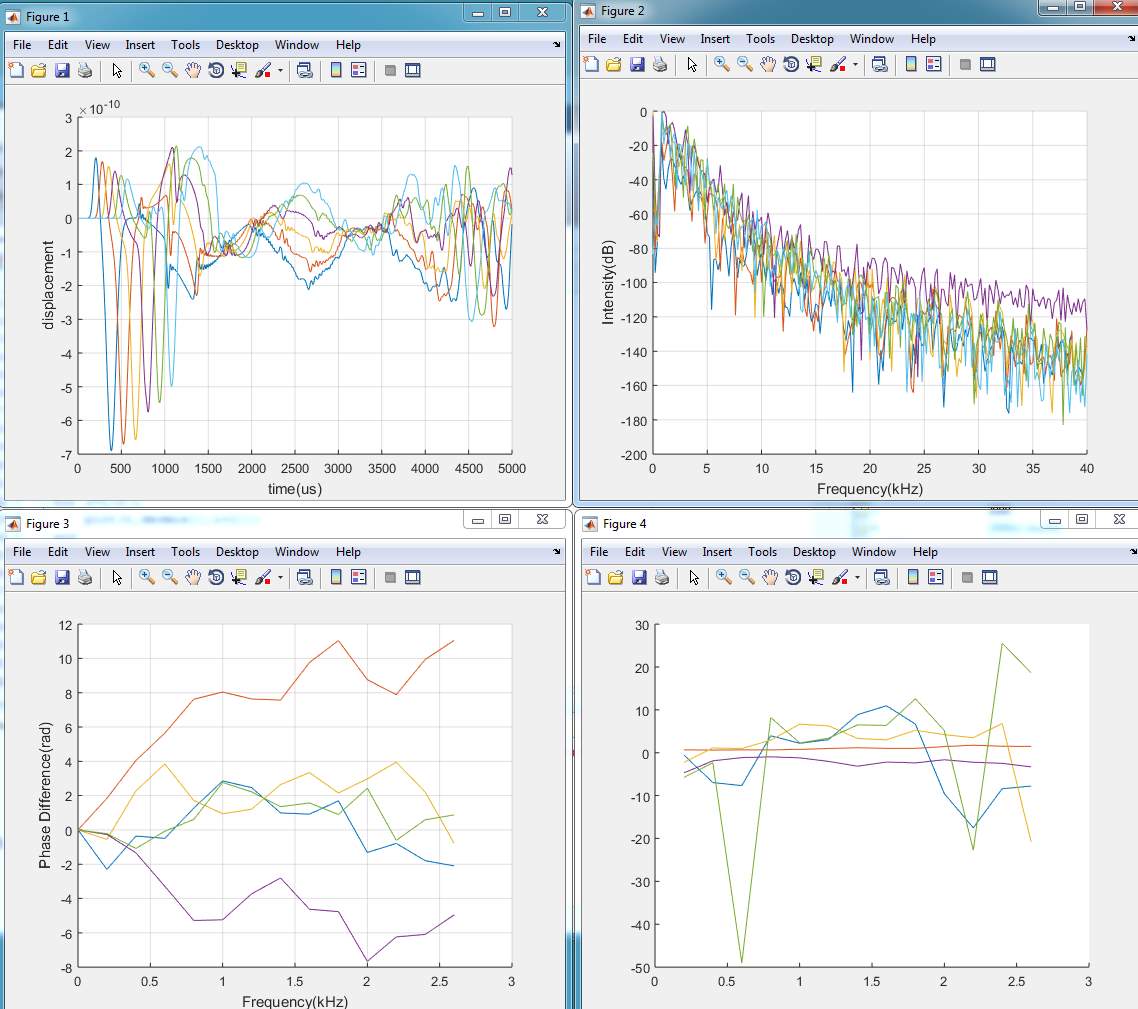
4. Model (D)
5. Model (E)
REFERENCES
Aki, K. and P. G. Richards. 1980. Quantitative Seismology: Theory and Methods. WH. Freeman and Company.
Aractingi, S., Aubin, F., Cribier, B., Joly, P. and Jullien, D., 2013. Risk of cancer in psoriasis: a systematic review and meta‐analysis of epidemiological studies. Journal of the European Academy of Dermatology and Venereology, 27(s3), pp.36-46.
Alkhorayef, M., Mahmoud , M., Alzimami, K. & Sulieman, A., 2015. High-Intensity Focused Ultrasound (HIFU) in Localized Prostate Cancer Treatment.. Issue 80, pp. 41-131.
Auld, B., 2014. Acoustic Fields and Waves in Solids. New York: John Wiley & Sons. Grate, J. W., 2012. Acoustic Wave Microsensor Arrays for Vapor Sensing Review. Issue 100., pp. 2627-2648.
Amirlak, B. 2015. Skin Anatomy: Overview, Epidermis, Dermis. Accessed at http://emedicine.medscape.com/article/1294744-overview
Breitkreutz, D., Koxholt, I., Thiemann, K. and Nischt, R., 2013. Skin basement Membrane: the foundation of epidermal integrity—BM functions and diverse roles of bridging molecules nidogen and perlecan. BioMed research international, 2013.
Betts, J. G., and others. 2016. OpenStax College, Anatomy and Physiology. OpenStax CNX. http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.26. McConnell, T.H. 2007. The nature of disease: Pathology for the health professions. Philadelphia: Lippincott Williams & Wilkins.
Chaussy, Christian G and Stefan Thüroff. 2011. Transrectal high-intensity focused ultrasound for local treatment of prostate cancer: current role. Arch. Esp. Urol. 2011; 64 (6): 493-506
Chan, R.C., Chau, A.H., Karl, W.C., Nadkarni, S., Khalil, A.S., Iftimia, N., Shishkov, M., Tearney, G.J., Kaazempur-Mofrad, M.R. and Bouma, B.E., 2004. OCT-based arterial elastography: robust estimation exploiting tissue biomechanics. Optics Express, 12(19), pp.4558-4572.
de Sousa Crespo, J., 2009. Human tissue hyperelastic analysis. Instituto Superior Tecnico, Universidade Tecnica de Lisboa.
Delalleau, A., Josse, G., Lagarde, J.M., Zahouani, H. and Bergheau, J.M., 2008. A nonlinear elastic behavior to identify the mechanical parameters of human skin
Diridollou, S., and others. 2000. In ‘vivo model of the mechanical properties of the human skin under suction’, Skin Res Technol 6(4), 214:221.
Fall, Dame et al. 2016. Surface acoustic wave characterization of optical sol-gel thin layers, Ultrasonics, Volume 68, May 2016, Pages 102-107, ISSN 0041-624X, http://dx.doi.org/10.1016/j.ultras.2016.02.006.
Goel, A. and others. 2016. Elastography, in radiopaedia.org. Accessed at https://radiopaedia.org/articles/elastography
Hashimoto, K., 2012. Surface Acoustic Wave Devices in Telecommunications: Modelling andSimulation. Berlin: Springer.
Heidenreich , A., Bastian, P. & Bellmunt, J., 2014. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013.. Issue 65, pp. 37-124.
HUEBNER, KENNETH H et al. 2001.The Finite Element Method for Engineers. Wiley-Interscience.
Holzapfel, G.A. 2000. ‘Biomechanics of soft Tissue’, Biomech Reprints Series. Austria: Gras University of Technology. 1-15.
In vivo. Skin Research and Technology, 14(2), pp.152-164.
Gennisson, J.L., Baldeweck, T., Tranter, M., Catheline, S., Fink, M., Sandin, L., Cornillon, C. and Querleux, B., 2004. Assessment of elastic parameters of human skin
Imokawa, G. and Ishida, K., 2015. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. International journal of molecular sciences, 16(4), pp.7753-7775
Kennedy, J. E. 2005. High intensity focused ultrasound in the treatment of solid tumors. Nature Reviews Cancers 5 (4) 321-327.
Kennedy, JE., Ter Haar, and G Cranston. 2003. High Intensity Focused ultrasound: Surgery of the Future? The British Journal of Radiology, 76: 590-599.
Kennedy, B.F., Hillman, T.R., McLaughlin, R.A., Quirk, B.C., and Sampson, D.D., 2009. In vivo dynamic optical coherence elastography using a ring actuator. Optics Express, 17(24), pp.21762-21772.
Kirkpatrick, S.J., Wang, R.K., Duncan, D.D., Kulesz-Martin, M. and Lee, K., 2006. Imaging the mechanical stiffness of skin lesions by in vivo acoustic-optical
Kalra A, Lowe A, Al-Jumaily AM (2016) Mechanical Behaviour of Skin: A Review. Journal of Material Science and Engineering. 5: 254. doi:10.4172/2169-0022.1000254
Leiter, U., Eigentler, T. and Garbe, C., 2014. Epidemiology of skin cancer. In Sunlight, Vitamin D and Skin Cancer (pp. 120-140). Springer New York.
Liang, X., Graf, B.W. and Boppart, S.A., 2011. In vivo multiphoton microscopy for Investigating biomechanical properties of human skin. Cellular and molecular bioengineering, 4(2), pp.231-238.
Li, C, and others. 2011. ‘Determining elastic properties of skin by measuring surface waves from an impulse mechanical stimulus using phase-sensitive optical coherence tomography’, Journal of the royal society Interface, 831-841.
Li, C. 2013. Quantitative assessment of elasticity properties of skin using surface acoustic wave (SAW) method. PhD: University of Dundee.
LIANG, X., CRECEA, V., & BOPPART, S. A. 2010. DYNAMIC OPTICAL COHERENCE ELASTOGRAPHY: A REVIEW. Journal of Innovative Optical Health Sciences, 3(4), 221–233. http://doi.org/10.1142/S1793545810001180
Moy, Austin. 2008. High Intensity Focused Ultrasound. Accessed at < http://bme240.eng.uci.edu/students/08s/amoy/physics.html>.
Chaussy, Christian G and Stefan Thüroff. 2011. Transrectal high-intensity focused ultrasound for local treatment of prostate cancer: current role. Arch. Esp. Urol. 2011; 64 (6): 493-506.
Maloney , E. & Hwang, J., 2015. Emerging HIFU applications in cancer therapy. InternationalJournal on Hyperthermia, Issue 31, pp. 9-302.
Menon, G.K., 2015. Skin Basics; Structure and Function. In Lipids and Skin Health (pp. 9-23). Springer International Publishing.
Michael, F. & Henaff, J., 2012. Surface Acoustic Waves for Signal Processing. Boston: Artech House.
Nakajima, M., Kiyohara, Y., Shimizu, M. and Kobayashi, M., 2007. Clinical application Of real-time tissue elastography on skin lesions. MEDIX Suppl, pp.36-39.
Napoli , A., Ahmed, M. & Peek, M., 2015. Systematic review of high-intensity focused ultrasound ablation in the treatment of breast cancer.. Issue 102, pp. 82-873.
Nicolle, S., Decorps, J., Fromy, B. and Palierne, J.F., 2016. New regime in the Mechanical behavior of skin: strain-softening occurring before strain-hardening. Journal of the Mechanical Behavior of Biomedical Materials.
Nguyen, T. M, et al. 2014. Visualizing ultrasonically induced shear wave propagation using phase-sensitive optical coherence tomography for dynamic elastography, Optics Letters, 39:4, 838-841.
Osmancevic, A., Gillstedt, M., Wennberg, A.M. and Larkö, O., 2014. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta dermato–venereologica, 94(4), pp.425-430.
Pouplard, C., Brenaut, E., Horreau, C., Barnetche, T., Misery, L., Richard, M.A.,
Quan, T. 2016. Molecular Mechanisms of Skin Aging and Age-Related Diseases. In Molecular Mechanisms of Skin Aging and Age-Related Diseases, ed. by. T. Quan. Boca Raton: Taylor and Francis Group, pp. 1:40.
Royer, D. & Dieulesaint, E., 2013. 2000. Elastic Waves in Solids (I & II),. Berlin: Springer.
Raveh Tilleman, T., Tilleman, M.M. and Neumann, H.A.M., 2004. The elastic properties of cancerous skin: Poisson’s ratio and Young’s modulus. Optimization of Incisions in Cutaneous Surgery including Mohs’ Micrographic Surgery., p.105.
SEER Training Modules, Skin Cancer: Melanoma. U. S. National Institutes of Health, National Cancer Institute. 08 February 2017 < https://training.seer.cancer.gov/melanoma/anatomy/layers.html >.
Tianjian, LU., and Feng, XU. 2008. MECHANICAL PROPERTIES OF SKIN: A REVIEW, Advances in Mechanics (4): 393-426.
Tomlins, P. H. and R K Wang. 2005. Theory, Development and application of Optical Coherence Tomography, Journal of Physics D: Applied Physics, 38:2519-35.
Wang, S., & Larin, K. V. 2015. Optical coherence elastography for tissue characterization: a review. Journal of Biophotonics, 8(4), 279–302. http://doi.org/10.1002/jbio.201400108
Weisstein, Eric W. “Finite Element Method.” From MathWorld–A Wolfram Web Resource. http://mathworld.wolfram.com/FiniteElementMethod.html
Wu, F., 2014. High intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancer. World J Gastroenterol, Issue 20.
Yoshida, K., Yokouchi, M., Nagao, K., Ishii, K., Amagai, M. and Kubo, A., 2013. Functional tight junction barrier localizes in the second layer of the stratum granulosum of human epidermis. Journal of dermatological science, 71(2), pp.89-99.
Zhang, X., Kinnick, R.R. and Greenleaf, J.F., 2008, November. Viscoelasticity of lung tissue with surface wave method. In Ultrasonics Symposium, 2008. IUS 2008. IEEE (pp. 21-23). IEEE.
TIME MANAGEMENT DIAGRAM
RISK ASSESSMENT


[1] Dame, F., Framcpos, C., Marc, D., Herve, P., Mohammadi, O., Frederic, J., . . . Chrystel, A. (2016). Surface acoustic wave characterization of Optical sol-gel thin layers. Ultranics, 102-107.
Gennisson, J., Baldeweck, T. T., Catheline, M. S., M, F., Sandrin, L., & Cornillon, C. (2004). Assessment of Elastic Parameters of hunman skin using dynamic elastography. IEEE transactions on ultrasonics, ferroelectrics, and frequency control, 908-989.
Li, C. (2013). Quantitative assessment of elasticity properties of skin using surface acoustic wave (SAW) method. 2-3.
[2] Elasticity: Ability of an object to return to its original size and shape when stress removed.
Poisson ratio: Ratio of lateral strain to axial strain.
Density: Mass per unit volume. (Wikipedia).
[3] (Li 2013)
[4] http://www.ansys.com/About-ANSYS.
[5] http://www.sp.phy.cam.ac.uk/research/fundamentals-of-low-dimensional-semiconductor-systems/saw
[6] http://earthquake.usgs.gov/learn/glossary/?term=Rayleigh%20wave
[7] 0.45-0.49 for Poisson’s ratio and 1000-1400 kg/m3 for density (Li 2013:50).
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medical Technology"
Medical Technology is used to enhance the medical care and treatment that patients are given in healthcare settings. Medical Technology can be used to identify, diagnose and treat medical conditions and illnesses.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: